Biology of nitrogen release from pulses
Biology of nitrogen release from pulses
Author: V S R Gupta Vadakattu, P Piana-Muschietti (CSIRO Agriculture & Food Waite Campus) and Lindsay Bell (CSIRO Agriculture & Food Toowoomba) and Therese McBeath (CSIRO Agriculture & Food Waite Campus) | Date: 25 Feb 2020
Take home messages
- All grain legume (pulse) crop residues generally have higher concentrations of nitrogen (N) and lower C to nitrogen ratio than cereal crops.
- The rate and timing of availability of N from pulse stubble to the following crops is determined by stubble decomposition rate and N immobilisation (tie-up) by soil micro-organisms.
- N mineralisation/tie-up processes after pulse crops can differ significantly to that after cereal and canola in terms of the (i) timing of release of N from residues (for example; during summer versus early into the following crop), (ii) magnitude of N tie-up during the seedling phase and (iii) the availability of fertiliser N to the cereal crop after a pulse crop.
- In low fertility soils, the combination of N release from pulse crops and N from fertiliser sources is required to maximise productivity benefits in the following crops.
Background
During the last decade, the effect of increasing intensification of cropping systems on the ability of grain legumes as rotational crops to sustainably reduce N inputs and increase N use efficiency (NUE) in non-leguminous crops following legumes has not been fully explored. It is generally considered that soil mineral N after both pasture and grain (pulses) legumes is higher than after cereals or canola crops. However, the magnitude of this benefit is found to vary for different legumes, especially for grain legumes (pulses) where it is strongly dependent on location and season. For example, Peoples et al. (2017) observed that on average the soil mineral N from legume crops was 35kg N/ha but varied from 11kg N/ha to 89kg N/ha based on experiments in eastern and southern Australia.
Stubble retention can provide benefits through changes in soil physical, chemical and biological properties. Soil biota play a key role in several essential biological functions where carbon (C) inputs from plant roots and crop residues form the essential supply of C (energy source) and nutrients for biological activities. Changes in the short-term flux of labile soil organic matter (SOM) pools (for example; dissolved OM and particulate OM due to stubble retention and crop rotation also influence biological N cycling and N availability. The concentrations of soil mineral N observed at the beginning of a growing season represent the net effect of different biological processes such as (i) residue decomposition, SOM cycling and N mineralisation by microbes, (ii) N tie-up by microbes, (iii) losses through leaching and gaseous losses and (iv) N uptake by weeds that either favour or reduce the accumulation of plant-available soil mineral N.
Therefore, in most Australian agricultural soils C inputs through above- (stubble) and below-ground (roots) plant residues have a major influence on populations of biota and their activities.
Recent research has indicated that cereal stubble mainly acts as a source of C for microbial activity thereby influencing N availability and may not be considered as a significant source of N to the following crops (Gupta et al. 2017). Our understanding of the fate of organic inputs from grain legume residues in terms of their decomposition, release of nutrients and soil OM build-up is yet to be fully explored. This paper presents a brief summary of what is known about the biology of pulse crop residues and N release in a cropping system.
Crop residues as a source of C and nitrogen
Crop residues (stubble) are one of the major inputs of OM into the soil and are the primary sources of C (energy source) for soil microorganisms (biota) both in agricultural and natural systems, particularly in low OM Australian soils. Stubble generally contains approximately 42% of C (dry weight basis) and a large portion of it is biologically available (labile) in the short-term (less than three years). Retention of stubble after harvest would also contribute to the conservation of nutrients that have been taken up by the plant within the cropping system. All plant residues are basically composed of the same primary building blocks (cellulose, hemicellulose, protein, lignin and lipids) albeit in different proportions, leading to the formation of SOM. For example, amino acids represent a key pool of C and N in soil, and their availability to plants and microorganisms has been implicated as a major driver in regulating biological functions such as SOM cycling and N transformations. Although plants also provide key inputs of belowground C and nutrient inputs through root exudation and turnover, aboveground stubble inputs are considered a major contributor of OM into the soil. With a harvest index of 34±2%, grain legume crops (pulses) add aboveground residues ranging from 1.4t/ha to 10t/ha which provides 1t of C/ha to 3.9t of C/ha for use by soil biota (Table 1). The amount of C inputs from stubble for faba bean and lupin was generally higher (averaging 2.1-3.1 tonnes of C/ha) and lower for lentil and mung bean crops (averaging 0.96-1.13 tonnes of C/ha).
The amount of nutrients in pulse stubble at harvest depends upon crop type, soil fertility, nutrition received through fertilisers and nutrients removed in the grain. Average concentrations of N in the stubble from different pulses are given in Table 1. The N content of harvest-shoot residues for different pulses are generally higher (0.8-1.2%) than for either cereal (0.4±0.06) or canola (0.7±0.06%) stubble. Therefore, pulse residues generally have lower C:N ratios (43:1 to 65:1) compared to that for cereal and canola residues (Table 1). At maturity, leaf material from legumes (generally representing 8-10% of residues) has a greater concentration of total N, soluble organic C and N and lower C:N ratio than stems. Additionally, N concentrations are highest for brown manure (BM) residues with the lowest C:N ratios compared to the same crops where grain was harvested. Previous research has shown that in addition to the aboveground residues, belowground nodulated roots also contribute a significant amount of C and N, which varies depending upon legume type and location (People et al. 2017; McNeill 1998, GRDC project UWA196WR). For example, belowground N at peak biomass of pulses grown at Goomalling in Western Australia (WA), measured using 15N labelling method, represented 40-50% of crop N for faba bean, lupin and chickpea crops. Overall, it was suggested that net inputs of legume N from the total crops at harvest ranged between 52-330kg N/ha for pulses (grain harvested) and 210-323kg N/ha for BM crops (Peoples et al. 2017).
Table 1. Comparison of estimates of above ground potential C and N inputs derived from crop (shoot) dry matter and C and nitrogen residue N remaining after individual legume (pulse) species grown for grain or brown manure (BM).
| Region | Legume crop | Shoot residues (t/ha) | Harvest Index | Carbon (%) | Nitrogen (%)1 | C:N ratio | C inputs (tonnes C/ha) | N inputs (kg N / ha) |
| NSW/SA/Vic (Peoples et al. 2017) | Field pea (n=7) | 3.70 (1.4-6.3) | 0.34 | 47.50 | 0.78 | 61 | 1.76 | 29 (11-49) |
| Chickpea (n=6) | 3.20 (1.9-4.6) | 0.30 | 47.50 | 0.86 | 55 | 1.52 | 27 (16-39) | |
| Lupin (n=6) | 4.60 (1.9-6.4) | 0.34 | 45.70 | 1.01 | 45 | 2.10 | 47 (19-65) | |
| Lentil/Vetch (n=2) | 2.40 (2.3-2.5) | 0.43 | 47.00 | 0.85 | 55 | 1.13 | 21 (20-21) | |
| Faba bean (n=5) | 5.30 (2.2-9.2) | 0.36 | 45.00 | 1.20 | 38 | 2.39 | 64 (26-110) | |
| Northern farming systems (Bell L, CSIRO; unpublished) | Field pea (n=5) | 4.69 (1.6-10.1) | 0.37 | 45.00 | 0.85 | 56 | 2.11 | 40 (14-86) |
| Chickpea (n=8) | 3.60 (1.2-6.5) | 0.37 | 47.50 | 0.86 | 55 | 1.71 | 31 (10-55) | |
| Faba bean (n=4) | 6.87 (4.7-9.2) | 0.39 | 45.00 | 1.05 | 43 | 3.09 | 72 (49-96) | |
| Mung bean (n=4) | 2.18 (1.4-2.4) | 0.20 | 44.00 | 0.80 | 55 | 0.96 | 17 (11-19) | |
| NSW/SA/Vic (Peoples et al. 2017) | Vetch BM3(n=2) | 5.25 (5.1-5.4) | 45.00 | 2.72 | 17 | 2.36 | 165 (139-147) | |
| Field pea BM (n=2) | 8.00 (6.3-9.8) | 47.50 | 2.30 | 21 | 3.80 | 223 (145-225) | ||
| Lupin BM (n=1) | 8.40 | 45.70 | 2.28 | 20 | 3.84 | 207 | ||
| Wheat2 | 1.00 | 0.35 | 45.00 | 0.42 | 107 | 0.45 | 4.2 | |
| Canola2 | 1.00 | 0.18-0.30 | 45.00 | 0.70 | 64 | 0.45 | 7.0 |
Notes: 1Percentage N values for legume stubble for the Northern Farming system experiments are estimated from published data;2Percentage N and harvest index values for wheat and canola are estimated from published data; 3No grain was harvested for BM crops, terminated with herbicide in mid-spring before grain maturity. Numbers in parenthesis represent range of values reported in the literature.
Since the growth and N fixation by legumes vary depending on crop season and soil type, care should be taken in estimating the amount of N benefit from the crop residues in specific fields and regions.
Decomposition of pulse stubble
Decomposition rates of legume residues is generally faster than that of cereal and canola residues (van Vliet et al. 2000). Results from a field experiment in South Australia (SA) indicated significant differences in mass loss of lupin (70%; decomposition rate k = 0.0045 % mass loss per day) and wheat (40%; decomposition rate k=0.0014 % mass loss per day), one year after harvest (Figure 1a & b). Similar findings were reported for lupin (20%) and wheat (15%) residue decomposition during summer months in WA (van Vliet et al. 2000). Differences in chemical composition in terms of soluble organic C and N; C:N ratio are considered the main contributors for the variation in decomposition rate, although other properties such as % cellulose, % lignin and polyphenol concentrations have also been shown to influence stubble decomposition. A general trend of reducing C:N ratio was seen in both the legume and wheat residues although C:N ratio of legume residues were always lower than that for wheat residues (Figure 1c).
In addition to residue quality, stubble decomposition and nutrient release patterns are influenced by climatic factors (especially moisture and temperature) and soil factors such as aeration, microbial biomass (MB) and nutrient status. Soil moisture influences microbial activity and N cycling processes near residues by directly affecting the microbial populations and their composition under water stress, and directly affecting the transport of dissolved organics and mineralised materials from residues. Indirectly, soil moisture influences soil aeration status. It has been reported that optimum moisture level of N mineralisation ranges between 45% to 60% water filled pore (WFP) space and at greater than 60% WFP denitrification can dominate due to sharp transition to O2-limiting conditions. The maximum decomposition rate of crop residues is generally reported to occur between 30°C to 35°C and minimum temperature for decomposition activity is approximately 5°C. Therefore, decomposition of crop residues and N mineralisation under Australian conditions is generally higher during summer (provided soil moisture is optimum) and lower during the winter season.
Interactions with microorganisms and management effects
Crop residue decomposition is mainly driven by soil microorganisms, although through direct and indirect interactions a complete food-web of soil organisms is involved. As soil microorganisms depend on residues for energy and cellular growth, stubble decomposition also affects soil microbial composition and activity. Soil fauna facilitate the decomposition of residues by acting as biocatalysers enhancing directly and indirectly the function of microorganisms through fragmentation and incorporation of stubble into soil, particularly in no-till systems.
Microorganisms responsible for stubble decomposition are diverse and their composition varies with residue quality, soil type and management. The level of MB is mostly higher with legume residues compared to that on wheat residues, especially during the early periods of decomposition (van Vliet et al. 2000). Significant differences in the composition of microbes were observed colonising legume stubble compared to wheat and canola residues (Figure 2). For example, colonisation by proteobacteria (fast growing microbes) was greater on legume residues (lower C:N ratio) compared to that on wheat residues (higher C:N ratio). The nutrient contents of pulse stubble are positively correlated with microbial activity and physiological diversity thereby influencing decomposition rate. Residue management in terms of incorporation technique, amount and time of incorporation can affect the composition and populations of microorganisms, thereby influencing decomposition rates. Incorporation of stubble and cutting it into small pieces increases the contact between stubble and soil microorganisms and the availability of soluble organic C and N. This modifies which type of microbes proliferate, and consequently the rate of decomposition and N release. Reduced tillage generally promotes greater proportion of fungi compared to incorporated stubble which promotes bacterial domination, with consequences to timing of N release.
Figure 1. The decomposition of lupin and wheat residues presented as biomass remaining as % of initial stubble residues in (a) field experiments at Karoonda, SA (Source: Muschietti-Piana, P, unpublished) and (b) field experiments at East Beverley, WA (Source: van Vliet et al. 2000) in litter bag experiments. (c) Residue C:N ratio in the Karoonda experiment and (d) changes in % N in the lupin and wheat residues during the six months of field incubation experiment at East Beverley, WA.
Nitrogen release - mineralisation and immobilisation
The release of N from crop residues and its availability to subsequent crops is a product of the rate of decomposition, mineralisation and immobilisation (microbial tie-up) processes. The conversion of organic N in the residues into inorganic N (nitrate N) available for plant uptake involves multiple biological processes mediated by different microbial functional groups. Some of these processes include residue decomposition (degradation of polyphenols and cellulose), proteolysis (conversion of proteins into amino acids) and nitrification (conversion of ammonium into nitrate). The abundance of different functional groups of N cycling microbes was found to be greater near legume residues and in soils following legume crops (Phillips et al. 2015; Gupta 2016). The net release of N from pulse residues with narrower C:N ratios is greater and quicker compared to that from cereal crop stubble. Also, net N mineralisation is greater from BM crops compared to grain harvested pulses (Peoples et al. 2017). However, the magnitude varies depending upon stubble load and agronomic factors such as tillage including the time of removal and type of stubble. For example, for BM crops the timing of crop termination during the growing season and its treatment would affect the decomposition, mineralisation and release of N into soil mineral N pool.

Figure 2. Photos of crop residues taken using a scanning electron microscope (SEM) showing the various types of microorganisms colonising stubble from vetch, canola and wheat crops. Extensive colonisation of nitrogen rich vetch residues by bacteria and fungi. Fungi and actinobacteria were the dominant microflora colonising wheat residues whereas fungal growth on canola residues was less and limited to specific fungi such as Pythium species.
Soil microbial biomass; the mass of living components of soil OM, is both a source and sink of biologically mediated nutrients. The amount of MB associated with residues (both legumes and cereal stubble) and especially in soil after legume crops is generally higher resulting in greater immobilisation of N (Figure 3). Immobilisation of N by MB during the early phases of decomposition has been observed with both legumes and cereal residues resulting in lower soil mineral N levels. Legume residues with lower C:N ratio immobilise to a lesser extent hence release N faster compared to that with cereal residues (Figure 1d). Fungi can translocate residue-derived C into the underlying soil while simultaneously translocating soil and fertiliser derived inorganic N up into the litter layer (Figure 1d). This stubble induced immobilisation could initially lower fertiliser N recovery efficiency by crops (Figure 2b), however N tied-up in MB is released with microbial turnover and due to faunal grazing of microbes, hence becomes available for crop uptake later in the season. Therefore, potential early N supply to crops could be improved by combining legume and fertiliser N, particularly in lower fertility soils (Muschietti-Piana et al. 2019).
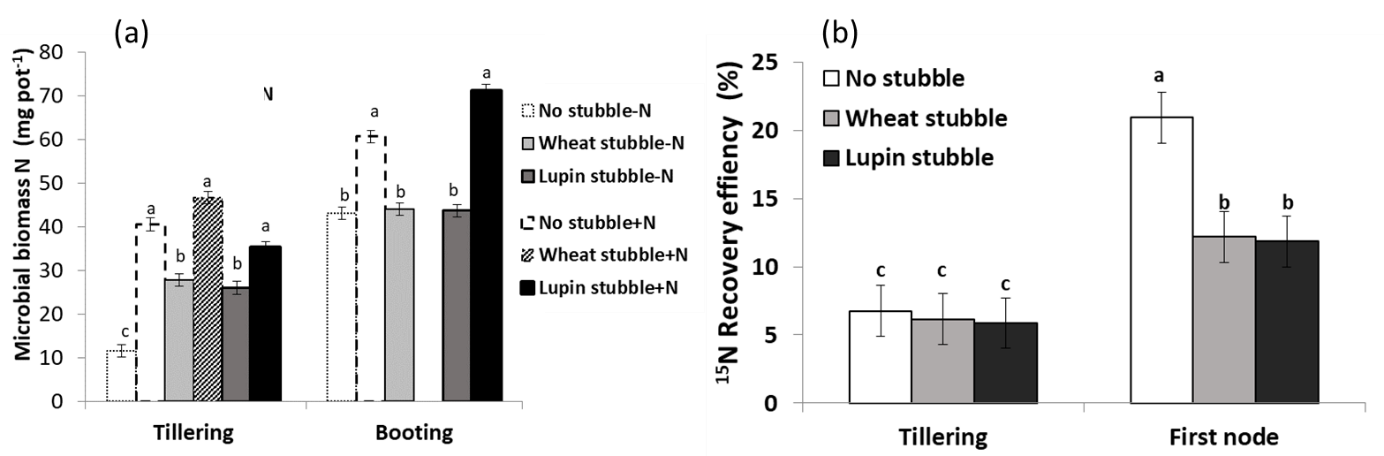
Figure 3. Effect of lupin and wheat stubble and N fertiliser application on (a) microbial biomass N in the surface 10cm soil at different growth stages. Within tillering, different letters indicate significant differences for the interaction between the ‘stubble treatment’ and ‘fertiliser N’ (DGC test, p<0.05). At booting, different letters indicate significant differences between treatment (DGC test, p<0.05). (b) 15N fertiliser recovery efficiency by wheat; different letters indicate significant differences for the interaction between ‘stubble treatment’ and ‘growth stage’ (DGC test, p<0.05).
Overall, the net release of N from legume residues is faster than that from cereal residues. This contributes to the general observation of higher mineral N in the soil profile at the end of the fallow period after legumes compared with cereal crops (Gupta 2016; Peoples et al. 2017). But due to the involvement of a multitude of factors related to residue, soil and the environment, the net release of N from crop residues, including pulses, could result in no observable increase in soil mineral N levels following summer months. For example, results from several Northern farming systems experiments during the 2015 to 2018 seasons indicated, no increase in soil profile mineral N levels following fallow after chickpea crops compared to that after wheat, in seven out of the 15 experiments (Bell L, GRDC project CSA00050). A wide range of pre-sowing soil mineral N values; 10-90 (35±20kg N) have been reported (Gupta 2016; Peoples et al. 2017). Nitrogen released from legume crop residues during summer months, when there is no plant uptake is less prone to leaching. Overall, apparent recovery of 30±10% of legume residue N by the following wheat crops was observed over 20 legume treatments in dryland experiments conducted in eastern Australia (Peoples et al. 2017). Whereas, cereal stubble is not a major source of N for following cereal crops and should be seen as a source of C for microbial activity. In no-till systems, only 1-6% of the N requirement of cereal crops is derived from the previous year’s wheat stubble (Gupta et al. 2017).
Although N concentration and C:N ratio of plant material is suggested as the most robust indices of residue quality, plant materials containing high concentration of recalcitrant C or lignin and polyphenols could also limit N mineralisation. The lateral roots and shoots of all legumes are expected to mineralise most rapidly given the low C:N ratios and less recalcitrant fractions, whereas wheat and canola shoots and wheat and lupin main roots might be expected to mineralise slower. Lignin to N and soluble polyphenols and polyphenol to N, polyphenol plus lignin to N ratios have also been suggested as indices of residue N release. Thus, no single index could characterise the quality of plant residues for their effect on all soil biological processes and N release.
Currently, there is very little information for decomposition rate and consequent N release rates (for example; temporal changes in mineralisation versus immobilisation) and the role of changes to soil microbiology for pulse residues in the different Australian grain growing regions, in particular under the intensive cropping and conservation agriculture systems currently practised.
Conclusion
Factors affecting N release after pulse crops include (i) amount of shoot residue biomass at the end of the growing season, (ii) N content and quality (e.g. C:N ratio) of the legume residues, (iii) environmental conditions; mainly rainfall over the fallow period before the next crop and (iv) soil microbial composition following the legume crop. The amount of aboveground plant biomass from different pulses not only limits the amount of C inputs for microbial activity but also limits potential N inputs from legume plant biomass. As the quality and N content of pulse crop residues defines the amount of N added to the system it consequently influences the N mineralisation and tie-up (immobilisation) processes. The rate and timing of the availability of N from pulse stubble to the following crop is determined by the rate of decomposition and immobilisation (tie-up) by the soil micro-organisms. However, the significance of these effects varies depending upon stubble load, time and type of burning and other agronomic factors.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support. The authors acknowledge the support provided by the GRDC (Projects MSF0003, CSP00186, CSA00050) and are grateful to the host landowners. Technical input of Bill Davoren, Willie Shoobridge, Stasia Kroker, Marcus Hicks is also thankfully acknowledged.
References
Gupta VVSR (2016) Biological factors influence N mineralisation from soil organic matter and crop residues in Australian cropping systems, Proceedings of the 2016 International Nitrogen Initiative Conference, "Solutions to improve nitrogen use efficiency for the world", 4 – 8 December 2016, Melbourne, Australia. Biological factors influence N mineralization from soil organic matter and crop residues in Australian cropping systems_CSIRO
Gupta VVSR et al. (2017) The effect of stubble on nitrogen tie-up and supply. GRDC Grains Research, Bendigo, Vic. GRDC_Bendigo_Update_Proceedings_2018_interactive.pdf
Phillips et al. (2015) Organic nitrogen cycling microbial communities are abundant in dry Australian agricultural soil. Soil Biology Biochemistry. 86: 201-211
Gupta VVSR et al. (2019) Harnessing the benefits of soil biology in conservation agriculture. In (Eds J Pratley and J Kirkegaard) “Australian Agriculture in 2020: From Conservation to Automation” pp 237-253 (Agronomy Australia and Charles Sturt University: Wagga Wagga)
Muschietti-Piana P et al. (2019) Improving early nitrogen supply to wheat by combining legume and fertiliser nitrogen. Journal of Plant Nutrition and Soil Science (in review).
Peoples MB et al. (2017) Soil mineral nitrogen benefits derived from legumes and comparisons of the apparent recovery of legume or fertiliser nitrogen by wheat. Soil Research 55, 600-615
Van Vliet PCJ et al (2000) Soil biota and stubble decomposition during summer and autumn in south-western Australia. Applied Soil Ecology.14: 111-124
Contact details
Dr. Vadakattu Gupta
CSIRO Agriculture and Food, Waite Campus, Urrbrae, SA 5064
08 83038579
Gupta.Vadakattu@csiro.au
@LifeInSoil5
GRDC Project Code: CSP1406-009RTX, CSP1406-007RTX, DAQ1406-003RTX,

