Septoria tritici blotch of wheat, management strategies for the medium and low rainfall zones of south east Australia
Septoria tritici blotch of wheat, management strategies for the medium and low rainfall zones of south east Australia
Author: Andrew Milgate (NSW DPI, Wagga Wagga Agricultural Institute) | Date: 11 Feb 2020
Take home messages
- Septoria tritici blotch (STB) has been building up in the farming system across south eastern Australia since 2010.
- Zymoseptoria tritici which causes STB has a long spore dispersal mechanism which allows for the spread of the disease from high rainfall to medium and low rainfall regions.
- Fungicide resistance to triazoles is ubiquitous however, active ingredients such as epoxiconazole and prothioconazole remain field effective at full rates.
- Wheat varieties with diseases ratings of moderately resistant- moderately susceptible (MRMS) reduce the need for fungicide application in medium rainfall environments.
- Risk of yield loss in medium and low rainfall environments is higher during above average rainfall years.
Background
Septoria tritici blotch (STB) of wheat is a necrotrophic disease caused by Zymoseptoria tritici. It is a disease of global importance and has recently been reported as the third most important disease to wheat production globally with losses ranked behind leaf rust and Fusarium head blight. The current epidemic of STB in south eastern Australia began in 2010 during numerous high rainfall years and an expansion of cropping in high rainfall zones (HRZ). This has created an environment where a large population of the pathogen survives each year to infect crops by wind dispersal in the high, medium (MRZ) and low (LRZ) rainfall environments when seasonal conditions are favourable. Outbreaks occur most years in the HRZ and were widespread during 2016 and 2017 in the Victorian and NSW medium/low rainfall zones and in Victoria/South Australia during 2019 across all rainfall zones.
Where is Septoria tritici blotch an issue?
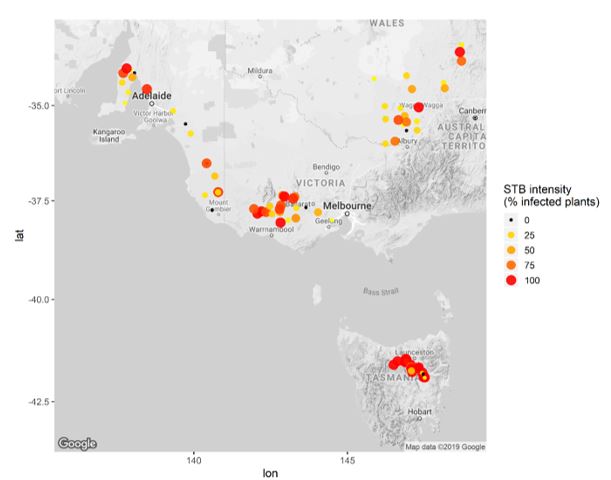
Z. tritici has a long-range spore dispersal mechanism, which means there is always a background level of inoculum present in most regions, surviving on stubble residues and susceptible varieties. The current distribution of STB in south eastern Australia, can be seen in recent paddock surveys conducted during the 2016 and 2017 seasons which found 78 out of 80 commercial wheat crops sampled had Z. tritici present (Figure 1).
Figure 1. Septoria tritici blotch infection intensity in 80 paddocks randomly surveyed across the medium and high rainfall zones of south eastern Australia during 2016-2017.
STB fungicide resistance in Australia
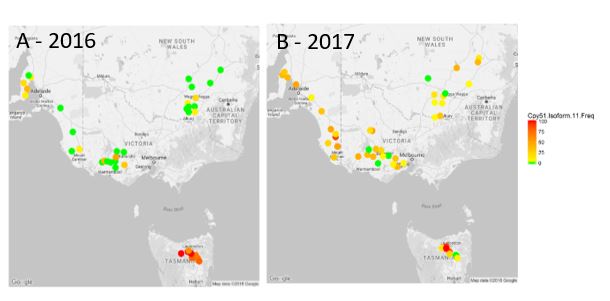
There has been rapid selection and spread of triazole fungicide resistance in the Australian Z. tritici population (McDonald et al., 2019). Figure 2 shows results of a STB fungicide resistance survey conducted by NSW DPI. In 2016 the highly resistant Cyp51 isoform G1 was found predominantly in Tasmania with only a few detections at low frequency on mainland Australia. This situation changed dramatically in 2017 as seen in Figure2b, where the G1 isoform was detected at most survey sites and its frequency had increased within paddocks. This isoform contains six mutations in the Cyp51 gene which encodes an altered protein structure (isoform) that significantly reduces the ability of fungicides, such as tebuconazole, propiconazole and flutriafol to bind with the target site and kill the fungus. Prior to 2016 there was no detection of this isoform outside of Tasmania. Its detection indicates long spore dispersal is occurring between regions, which allows the rapid migration of new forms of resistance throughout south eastern Australia. Other isoforms of the Cyp51 are present in Australia but at this stage they do not cause higher levels of fungicide resistance. While our current knowledge indicates that only the triazole fungicides have been affected thus far, the increasing use of succinate dehydrogenase inhibitor (SDHI) fungicides and strobilurins means it is only a matter of time before resistance to these active ingredients is observed in Australia, as has been the case in Europe. This means reliance on fungicides alone as a control strategy is likely to fail. In the European Z. tritici population, the G1 isoform has been superseded by isoforms with even higher levels of triazole resistance.
Figure 2. Map of occurrence of Cyp51 Isoform G1 in southern eastern Australia in a) 2016 and b) 2017. Green (predominantly mainland and decreasing in prevalence from 2016 to 2017) indicates absence of the G1 isoform (0%) through orange (increasing in prevalence from 2016 to 2017) to red (predominantly Tasmania) indicates G1 isoform detected in every sample from a paddock (100%).
Variety resistance available
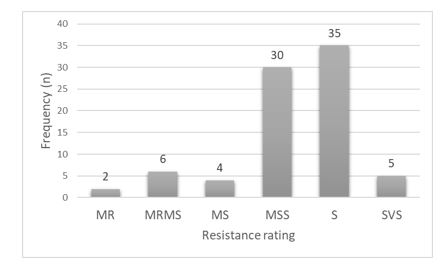
Variety resistance levels listed on the National Variety Trial (NVT) website show only eight varieties that are rated better than moderately susceptible (MS) out of 82 varieties currently on the market and tested in the NVT system. The most widely grown varieties in the medium and high rainfall areas across the eastern states are rated worse than MS. Which creates a large area of crop sown to susceptible cultivars allowing pathogen survival every year and crops vulnerable to disease epidemic development. Recent popular varieties such as Trojan, Mace and Scepter are all rated susceptible to current STB populations across eastern Australia. In the past varieties such as Yitpi provided good levels of adult plant resistance which helped reduce the inoculum levels across the medium rainfall zone in SA and parts of Victoria, thus reducing the risk of yield loss. In medium rainfall environments increasing the area sown to varieties with resistance better than susceptible ratings has a long-term positive effect on disease control.
Figure 3. Frequency of National Variety Trial disease resistance ratings to STB for 2019 of 82 commercially available wheat varieties. MR – Moderately resistant, MRMS – Moderately resistant moderately susceptible, MS- Moderately susceptible, MSS – Moderately susceptible to susceptible, S- Susceptible, VS- Very susceptible.
Integrated disease management (IDM) for STB in medium rainfall environments
Decisions to manage STB in medium rainfall regions are more complex than in high rainfall regions. The practice of early sowing and stubble retention increases the chance of infection by STB. This is because the primary source of inoculum is retained in the farming landscape and early sowing synchronises the availability of susceptible hosts with ascospore release from the stubble. Growers are then reliant on fungicides to avoid yield losses. However, in contrast to high rainfall regions the return on using fungicides in medium rainfall regions is lower and or non-existent. This is because while infection levels can be high from seedling through to flag leaf (GS39), conditions for disease progression to continue during the booting to grain fill phase are not as frequently met. To follow are some principals to help guide practices for both fungicide resistance management and crop management:
Fungicide resistance and disease management
To achieve fungicide resistance management and disease management there are three important steps growers need to implement:
1. Stubble removal.
- Stubble is the source of the infection each year. By removing stubble before sowing there is a substantial reduction of pathogen population size.
- Reduces all isoforms irrespective of resistance and reduces the initial establishment of disease.
- To be effective, the removal must reduce infected stubble to very low levels, ideally below 100kg/ha of infected stubble remaining within a paddock.
- Do not sow wheat on wheat.
- Variety choice.
- Under high disease pressure a variety rated MRMS can reduce the leaf area loss by as much as 60% compared to a susceptible – very susceptible variety (SVS).
- Host resistance reduces all isoforms irrespective of resistance and reduces the need for multiple canopy fungicide applications.
- Resistance ratings do change, so crops must still be monitored in-season for higher than expected reactions and each year it’s necessary to check for updates to disease ratings.
2. Fungicide choice and use.
- Do not use the same triazole active ingredient more than once in a season. Do not use a strobilurin or SDHI more than once in a season.
- Aim for early control of disease. STB spreads up the leaf layers of the canopy through rain splash and direct leaf contact. Reducing the disease in the lower canopy slows the upward movement of disease and ultimately the leaf area lost.
- Follow label instructions at all times.
- Timing of application in the disease epidemic period is critical for getting the most out of these products.
Integrated disease management
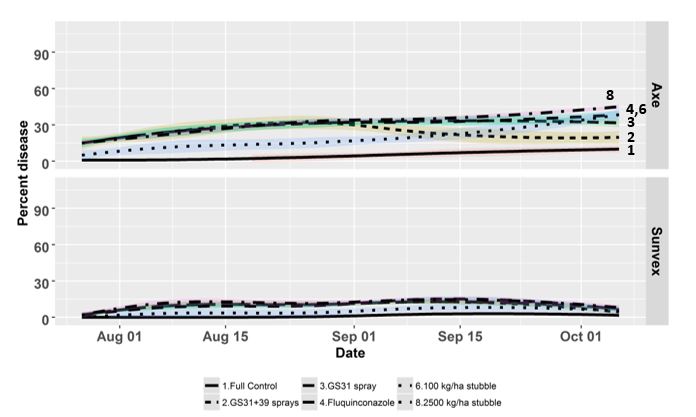
Figure 4 illustrates the benefits of combining variety resistance, fungicides and reducing the amount of stubble inoculum for the percentage reduction in disease. In this scenario, two varieties are contrasted, Axe which is S to STB and Sunvex which is MRMS to STB grown at Wagga Wagga in 2017 under dryland conditions.
Figure 4 shows the disease development within the canopy over time in six treatments. For Axe there are obvious reductions in disease with regular application of fungicides in the protected treatment. However, when fungicides are applied only at GS31 under high disease pressure little impact is visible and the disease level remains high, similarly for the upfront treatment of fluquinconazole. Whereas when a two-spray program at GS31+GS39 is applied, disease control improves. Note that the effect of reducing inoculum in a S variety has little impact on disease progress. When the effect of the same treatments is examined with Sunvex which has higher resistance the impact is very different. In this situation the benefits of fungicide applications are less obvious, and reduction of stubble inoculum has a similar impact to the application of fungicides. This example shows that growers should look to use multiple strategies to reduce disease in their farming systems because the application of fungicides alone does not always result in improved yield outcomes in all varieties and in all years.
Figure 4. Trial grown at Wagga Wagga, NSW 2017. Displayed are a selection of varieties and treatments from the trial. The whole trial contained five varieties and eight treatments. The varieties displayed here are Axe and Sunvex. The treatments displayed here are 1. Full control – multiple applications of propiconazole at 500ml/ha at 250 g/l active ingredient. 2. High disease pressure (2.5 t/ha stubble) with fungicide applied at GS31 and GS39. 3. High disease pressure with fungicide applied at GS31. 4. High disease pressure and fluquinconazole applied to the seed. 6. Low disease pressure (100 kg/ha stubble) 8. High disease pressure (2.5 t/ha stubble).
Conclusion
Understanding the lifecycle of STB presents opportunities for growers to be proactive about fungicide resistance and disease control. STB is a pathogen that survives from one crop to the next on the stubble left after harvest. An ideal time to take action is during this period, which should reduce the overall disease burden. In southern eastern Australia it is expected that infections of STB, particularly in the higher rainfall areas, will increase and these will require action by growers to prevent losses. However, in the medium rainfall areas early infections of crops are likely to occur but without above average rainfall from August to October these infections will present lower threats to the yield potential of crops.
Acknowledgements
The research undertaken here is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support.
Members of the cereal pathology team at the Wagga Wagga Agricultural Institute (WWAI) who contribute to the fungicide resistance work; Melanie Renkin, Merrin Spackman and Beverly Orchard. Project partners Megan MacDonald and Peter Solomon at Australian National University. Samples submitted to the survey by agronomists and growers across the HRZ, MRZ and LRZ throughout south eastern Australia during 2016 and 2017.
Useful resources
Septoria tritici blotch experiments in southern NSW 2015, pp 148-150 in Southern NSW research results 2015.
References
McDonald, M. C., Renkin, M., Spackman, M., Orchard, B., Croll, D., Solomon, C. P. S., & Milgate, A. (2019). Rapid Parallel Evolution of Azole Fungicide Resistance in Australian Populations of the Wheat Pathogen Zymoseptoria tritici. Applied and Environmental Microbiology, 85(4). doi:UNSP e01908-18 10.1128/AEM.01908-18
Contact details
Dr. Andrew Milgate
WWAI, Pine Gully Rd, Wagga Wagga, NSW, 2650
02 69381990
Andrew.milgate@dpi.nsw.gov.au
Was this page helpful?
YOUR FEEDBACK