Can wild species of chickpea from Turkey help with resistance to root-lesion nematode (Pratylenchus thornei)?
Take home messages
- This is the first investigation of a new collection of wild germplasm for nematode resistance. It offers the chance to exploit novel sources of P. thornei resistance and untapped genetic diversity, currently not available in cultivated chickpea and will be valuable for national and international chickpea breeding programs
- Accessions of the wild chickpea species (Cicer reticuatum and C. echinospermum) from Turkey, were on average more resistant to the root-lesion nematode Pratylenchus thornei than commercial cultivars of chickpea (C. arietinum)
- A total of 53 (30%) wild accessions were found significantly more resistant to P. thornei than the least susceptible Australian chickpea cultivar PBA Seamer
- Thirteen of the wild accessions were more resistant than a wild relative of chickpea, C. echinospermum ILWC 246 which was identified from earlier studies when only a limited number of wild accessions were available
- Having novel sources of P. thornei resistance with possible resistance to multiple diseases and insect pests such as cotton boll worm (Helicoverpa armigera), will increase the base of P. thornei resistance, and genetic diversity within chickpea for deployment in chickpea breeding programs aimed at developing new varieties with improved yield but possessing P. thornei resistance
- Linking the data with genetic diversity studies can provide information on P. thornei resistance genes and their locations in the chickpea genome
- Having more effective genes for resistance to P. thornei in chickpea cultivars will protect chickpea from yield loss and result in lower P. thornei residual populations in the soil. This will benefit other susceptible crops such as wheat, resulting in more flexible rotations with a profitable legume, and allow increased profit for growers.
Background
Wild chickpea and their role in developing cultivars to combat Pratylenchus thornei in the Australian grain region.
Crop wild relative (CWR) species are a rich source of genetic disease resistance and diversity and play a major role in meeting disease and environmental challenges in 21st century agriculture. Currently, there is limited genetic diversity within chickpea (Cicer arietinum) germplasm, and this hinders further progress in plant breeding worldwide to combat diseases and environmental stresses (Croser et al., 2003). Due to global changes, wild relatives are under threat of disappearing, and wild collections such as the one studied here are economically vital for the future of chickpea breeding and biodiversity in agriculture crops (von Wettberg et al., 2018). The chickpea genus Cicer has 43 wild species but only two wild annuals, Cicer reticulatum and C. echinospermum, can be hybridised with cultivated chickpea. These two wild species occur only in south eastern Turkey where chickpea originated as a domestic crop some 10,000 years ago.
The root-lesion nematode (RLN), Pratylenchus thornei, also known as the cereal and legumenematode,is a microscopic eelworm found in 67% of paddocks within the northern grain region of Australia (Thompson et al., 2010). It is the dominant species causing damage in chickpea crops throughout Australia, Europe and Asia with yield losses estimated up to 25% for intolerant Australian chickpea cultivars, and 58% in western Asia (Zwart et al., 2019).
Management for RLN relies on rotation with tolerant and resistant crops, however, P. thornei has a wide host range and several seasons of resistant crops are required to reduce soil populations below damaging levels of 2,000 per kg of soil (equivalent to 2 per gram of soil) (Owen et al., 2014). Chemical control is non economical, and inefficient in killing nematodes at depth in the soil profile (Reen et al., 2014). Furthermore, in dry periods, the nematodes are capable of going into a state of anhydrobiosis during slow dehydration of the soil and can become active again once soil moisture is restored. The long term, environmentally sustainable and cost effective solution to the problems with P. thornei is to breed and grow crop cultivars with resistance.
The terms resistance and tolerance are often used indiscriminately but both mean two very different things.
- Tolerance is how well a crop yields in the presence of nematodes and growing a tolerant cultivar will help minimise yield loss. However, tolerant cultivars while suffering less yield loss do not reduce nematode soil populations, unless they also possess resistance, and consequently will keep building up soil populations of P. thornei thereby limiting rotational options
- Resistance refers to how much a crop cultivar will limit nematode multiplication within the crop roots and soil, thereby limiting the damaging effects on that crop and reducing residual P. thornei soil populations to allow more flexible choice of crop rotations.
Crop cultivars can vary in their levels of resistance and tolerance, and breeding programs aim at incorporating a combination of both traits. This has been beneficial in wheat where the combination of resistance and tolerance results in higher yields compared to cultivars being tolerant and susceptible (Sheedy et al., 2012). Chickpea tend to be more tolerant than its main rotational crop wheat, however, current commercial cultivars range from moderately susceptible to very susceptible and will keep building up P. thornei populations in the soil.
Previous research seeking P. thornei resistance in C. arietinum has been extensive, but little resistance was found in cultivated germplasm. The search for useful traits in chickpea has been hindered by the narrow genetic diversity in cultivated chickpea, and a previously limited world collection of wild chickpea that consisted of only 18. C. reticulatum and 10 C. echinospermum accessions (Berger et al., 2003). Recent collection missions in Turkey in 2013 have boosted the numbers of wild accessions. This new 2013 collection has 100 times more genetic diversity than cultivated chickpea and 12 different genetic population groups were identified within the collection (von Wettberg et al., 2018). This new, larger collection is the focus of this study and the aim was to identify wild accessions with superior P. thornei resistance that could be used for breeding Australian cultivars.
Material and methods
The wild chickpea accessions originated from 21 collection sites within five provinces of Turkey and were tested twice for resistance to P. thornei over a two year period in controlled glasshouse studies. In order to determine the genetics controlling resistance, it is important to carry out these studies without any environmental influences that can mask the resistance. Extensive research has shown studies carried out in controlled glasshouse environments to assess resistance to P. thornei accurately predict field resistance (Thompson et al., 2019).
A total of 174 wild chickpea accessions (133 C. reticulatum, 41 C. echinospermum) plus twenty-two reference cultivars that ranged in levels of P. thornei resistance were tested. The reference cultivars included 11 Australian desi chickpea cultivars that were moderately susceptible (PBA Boundary ,PBA HatTrick, PBA Seamer, PBA Pistol, Flipper, Howzat and Yorker), susceptible (Jimbour, Sona, and Sonali) and very susceptible (Kyabra) plus a number of breeding lines with wild relative backgrounds. Four other reference wild Cicer accessions were included consisting of one resistant and one susceptible C. reticulatum (ILWC 123, ILWC 184 respectively), and one resistant and one moderately susceptible C. echinospermum (ILWC 246, ILWC 39 respectively). Plants were grown for 18 weeks and nematodes extracted from the roots and soils and counted under a microscope. There was a high correlation between experiments (0.84%) so data was analysed across the two years to obtain genetic rankings of resistance for the accessions.
Summary of results
On average, the wild species were more resistant than domesticated chickpea cultivars (C. arietinum Table 1). While there was a range of resistance within the wild species, neither species appeared more resistant than the other.
Genetic rankings showed (13) 7% wild accessions were more resistant than the most resistant C. echinospermum reference ILWC 246 from previous studies (Thompson et al., 2011).
A further 40 accessions (23%) were significantly more resistant than the least susceptible Australian chickpea cultivar PBA Seamer (Figure 1). This range of resistance for all accessions is illustrated in Figure 2 and shows how the wild accessions are more to the resistant end of the scale. Furthermore two accessions with high P. thornei resistance also have promising cotton bollworm resistance (Helicoverpa armigera) (Von Wettberg et al., 2018).
Mean P. thornei numbers also differed for collection sites and genetic population groups. There were no obvious trends for association of P. thornei resistance in terms of elevation and geographic location, as resistant accessions occurred at all elevations and at all sites.
Table 1. Population densities of Pratylenchus thornei in relation to Cicer species. Means are derived from best linear unbiased estimators (P. thornei per kilogram of soil + roots) in Experiments 1 and 2.
Experiment | Species | No. Accessions | P. thornei/kg of soil + rootsy | |
|---|---|---|---|---|
Loge | Mean | |||
1 | C. arietinum | 11 | 10.13 a | 25,804 |
C. echinospermum | 34 | 8.61 b | 5,503 | |
C. reticulatum | 121 | 8.59 b | 5,359 | |
2 | C. arietinum | 12 | 10.42 a | 33,390 |
C. reticulatum | 135 | 9.38 b | 11,897 | |
C. echinospermum | 43 | 9.35 b | 11,487 | |
Values followed by the same letter within each experiment are not significantly different (P < 0.05). Modified from Reen et al 2019.
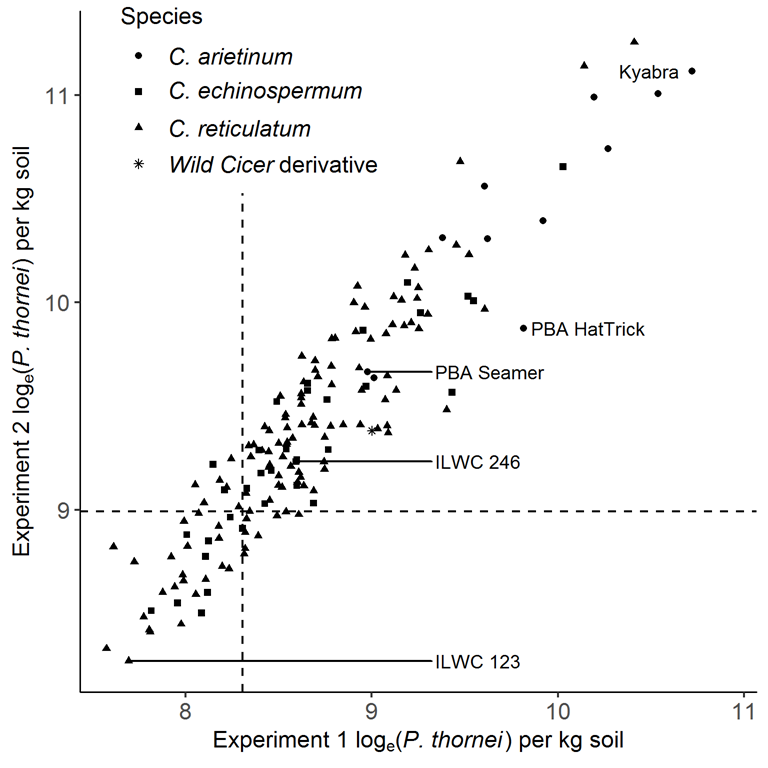 Figure 1. Accession means (best linear unbiased predictors) of Pratylenchus thornei population densities for Cicer accessions included in both experiments showed a strong genetic correlation between experiments (r = 0.844, n = 167). Vertical and horizontal dashed lines denote the cut-off points for the top 20% accessions for resistance to P. thornei. (Source: Reen et al., 2019).
Figure 1. Accession means (best linear unbiased predictors) of Pratylenchus thornei population densities for Cicer accessions included in both experiments showed a strong genetic correlation between experiments (r = 0.844, n = 167). Vertical and horizontal dashed lines denote the cut-off points for the top 20% accessions for resistance to P. thornei. (Source: Reen et al., 2019).
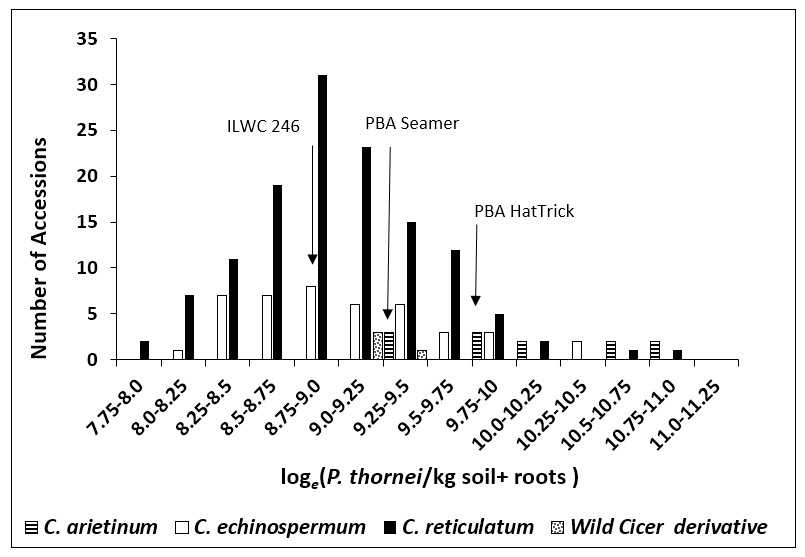
Figure 2. Distribution of accessions showing the spread of resistance to P. thornei for domesticated chickpea cultivars (C. arietinum) and wild chickpea accessions (C. echinospermum & C. reticulatum). Results are expressed in log e (P. thornei / kg soil) from combined analysis of two experiments where accessions were tested twice. Accessions left of PBA Seamer have lower values and therefore higher resistance to P. thornei than domestic cultivars. ( Source from Reen et al 2019).
Discussion
The success of chickpea crop improvement is reliant on the amount of genetic variation available within the germplasm, and investment in new collections such as those studied here are valuable for chickpea breeding worldwide. Wild chickpeas possess a diversity of adaptive genes to disease stresses that have evolved over thousands of years independent of domestication (Berger et al., 2003).
This research is the first worldwide to assess nematode resistance for this new collection of wild relatives of chickpea. The results reveal the collection contains new and diverse sources of P. thornei resistance and untapped genetic diversity, valuable for Australian and international chickpea breeding programs to exploit. Furthermore, the identification of P. thornei resistance within this collection, highlights the crucial importance of wild species for meeting the challenges in relation to biotic stresses facing the industry today.
Where to from here?
Targeting and exploiting accessions with Pratylenchus thornei resistance identified in this research, provides an excellent opportunity to harness this diversity and resistance for chickpea improvement. Within this wild collection, 26 diverse accessions were selected internationally as parents for breeding with elite chickpea cultivars that represent major growing and climatic regions of the world. In Australia, this elite cultivar was PBA HatTrick, and five resulting hybrid populations with improved P. thornei resistance have been selected for advancement and mapping studies to identify P. thornei resistance genes for future breeding purposes.
It is hoped, future assessment of the resulting progeny combined with genomic resources will facilitate more rapid development of P. thornei resistant cultivars with the possibility of combined resistance to multiple diseases. The results also provide the basis for further studies to improve knowledge about the genetic control of P. thornei resistance in chickpea. It is anticipated in future research, to pyramid the resistant genes with the combining of multiple resistance to other stresses. The outcome for the chickpea industry will be a reduction in P. thornei populations in the soil, which will not only benefit chickpea but other susceptible crops such as wheat, resulting in more flexible crop rotations and increased yields and whole farm profitability.
Acknowledgments
The research undertaken in this project was possible by significant contributions of growers and support of the GRDC, and by DAF Queensland and USQ under the Broad-acre Cropping Initiative. The authors would like to thank them for their continued support.
Furthermore, thanks to AGG for supplying the wild Cicer collection and Professor Doug Cook, UC Davis, California, and Dr Jens Berger CSIRO Australia, for collection and importation of the wild Cicer accessions into Australia. Special thanks to Hannah Rostad (USQ), for technical assistance, and Kerry Bell in statistical support (QDAF).
References
Berger JD, Abbo S & Turner NC (2003) Ecogeography of annual wild Cicer species: The poor state of the world collection. Crop Science 43, no. 3, 1076-1090.
Croser JS, Ahmad F, Clarke HJ & Siddique KHM (2003) Utilisation of wild Cicer in chickpea improvement; progress, constraints, and prospects. Australian Journal of Agricultural Research 54, no. 5, 429-444.
Owen KJ, Clewett TG, Bell KL & Thompson JP (2014) Wheat biomass and yield increased when populations of the root-lesion nematode (Pratylenchus thornei) were reduced through sequential rotation of partially resistant winter and summer crops. Crop and Pasture Science 65, no. 3, 227-241.
Reen RA, Thompson JP, Clewett TG, Sheedy JG & Bell KL (2014) Yield response in chickpea cultivars and wheat following crop rotations affecting population densities of Pratylenchusthornei and arbuscular mycorrhizal fungi. Crop and Pasture Science 65, no 5. 428-441.
Reen RA, Mumford MH, Thompson JP (2019) Novel sources of resistance to root-lesion nematode (Pratylenchus thornei) in a new collection of wild Cicer species (C. reticulatum and C. echinospermum) to improve resistance in cultivated chickpea (C. arietinum). Phytopathology 109, 1270-1279.
Sheedy JG, Thompson JP & Kelly A (2012) Diploid and tetraploid progenitors of wheat are valuable sources of resistance to the root lesion nematode Pratylenchus thornei. Euphytica 186, no. 2. 377-91.
Thompson JP, Clewett TG, Sheedy JG, Reen RA, O’Reilly MM & Bell KL (2010) Occurrence of root-lesion nematodes (Pratylenchus thornei and P. neglectus) and stunt nematode (Merlinius brevidens) in the northern grain region of Australia. Australasian Plant Pathology 39, no. 3, 254-264.
Thompson JP, Reen RA, Clewett TG, Sheedy JG, Kelly AM, Gogel BJ & Knights EJ (2011) Hybridisation of Australian chickpea cultivars with wild Cicer spp. increases resistance to root-lesion nematodes (Pratylenchusthornei and P. neglectus). Australasian Plant Pathology, 40, 601-611.
Thompson JP, Sheedy GJ and Robinson NA (2019) Resistance to wheat genotypes to root-lesion nematode Pratylenchus thornei can be used to predict final nematode densities, crop greenness and grain yield in the field. Phytopathology.
von Wettberg EJB, Chang PL, Başdemir F, Carrasquila-Garcia N, Korbu LB, Moenga SM, Bedada G, Greenlon A, Moriuchi KS, Singh V, Cordeiro MA, Noujdina NV, Dinegde KN, Shah Sani SGA, Getahun T, Vance L, Bergmann E, Lindsay D, Mamo BE, Warschefsky EJ., Dacosta-Calheiros E, Marques E, Yilmaz MA, Cakmak A, Rose J, Migneault A, Krieg CP, Saylak S, Temel H, Friesen ML, Siler E, Akhmetov Z, Ozcelik H, Kholova J, Can C, Gaur P, Yildirim M, Sharma, Vadez V, Tesfaye K, Woldemedhin AF, Tar’an B, Aydogan A, Bukun B, Penmetsa RV, Berger J, Kahraman A, Nuzhdin SV and Cook DR (2018). Ecology and genomics of an important crop wild relative as a prelude to agricultural innovation. Nature communications 9, Article 649.
Zwart RS, Thudi M, Channale S, Manchikatla PK, Varshney RK & Thompson JP (2019), Resistance to plant-parasitic nematodes in chickpea: Current status and future perspectives. Frontiers in Plant Science 10, no. 966.1-14
Contact Details
Roslyn Reen
Centre for Crop Health
University of Southern Queensland
Ph: 07 4631 1208
Email: Roslyn.reen@usq.edu.au
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
GRDC Project Code: USQ00017,
Was this page helpful?
YOUR FEEDBACK
