Herbicide resistance survey results of the Northern cropping region
Herbicide resistance survey results of the Northern cropping region
Author: Adam Jalaludin (Department of Agriculture and Fisheries), Michael Widderick (Department of Agriculture and Fisheries), John Broster (Charles Sturt University), Allison Chambers (Charles Sturt University) and Michael Walsh (University of Sydney) | Date: 21 Jul 2020
Take home message
- Glyphosate resistant weeds are present in the northern region. Glyphosate failed to control all of the fleabane populations tested. Glyphosate resistance was also prevalent in feathertop Rhodes grass, windmill grass and awnless barnyard grass, with resistance detected in 68%, 58% and 36% of populations, respectively. Only 14% of sowthistle populations were resistant to glyphosate
- Evolved herbicide resistance to haloxyfop was also detected in feathertop Rhodes grass, albeit at a low frequency
- Other herbicides such as 2,4-D amine, propaquizafop and clethodim provided good control of the broadleaf and grass weeds tested
- Farmers and agronomists should incorporate non-chemical weed management tactics to ensure sustainability of current herbicides
- These survey results provide a first glimpse into the state of herbicide resistance in key crop weeds for Queensland and the Northern region.
Introduction
The area of the Northern grain cropping region from Central Queensland to Dubbo in New South Wales (north to south) has a diverse cropping system, often with both winter and summer cropping, or summer fallow for moisture storage. Effective weed control in crop and fallow is heavily reliant on herbicides such as glyphosate. For the past 30 years, heavy reliance on glyphosate has seen more and more weed species become resistant to this herbicide. This includes annual ryegrass, awnless barnyard grass, liverseed grass, windmill grass, fleabane and sowthistle (Cook 2014, Widderick et al. , 2014, Cook et al. 2004).
Despite the increasing number of herbicide resistance cases, the full extent of herbicide resistance in this part of the Northern region is unknown. The first whole of region survey of key winter and summer weed species started in winter 2016 and was completed in summer 2017/2018. Weed seeds collected from the survey were screened for resistance against various herbicides. This report primarily covers the herbicide resistance screening carried out by the Department of Agriculture and Fisheries, Queensland.
Material and methods
The Department of Agriculture and Fisheries, Queensland (DAF) was responsible for herbicide resistance screening of sowthistle, fleabane, barnyard grass, feathertop Rhodes grass, windmill grass and liverseed grass. Populations of the species listed above that were collected in New South Wales by Dr John Broster of Charles Sturt University were also screened by DAF.
Seed germination
Weed seeds were grown in their growing seasons following the survey. Sowthistle were grown from late March to late November while fleabane and the summer grasses were grown from late November to mid-May.
Sowthistle and summer grass seeds were germinated on 0.6% agar for 4 to 7 days in the glasshouse and transplanted into trays (50 seedlings/population/tray) of potting mix. The trays were then placed onto drip trays filled with enough water to keep the soil moist without causing any water logging for the first few days. After 7 days, the plants were watered from above. A similar approach was used for fleabane, with the exception that fleabane seeds were sown directly into trays of potting mix. After 7 days, fleabane seedlings were thinned to the required density (10 seedlings per column, up to 5 columns).
Herbicide resistance screening
Plants were grown until they reached the three to five leaf growth stage. They were then treated at the recommended label rate for each herbicide (Table 1) with the appropriate adjuvant (if required). For weeds that are not on label for a particular herbicide, the recommended rate for the closest relative weed was used. Assessment of survivors was carried out at 21 days after treatment (DAT). Plants were considered surviving if there were actively growing tillers (grass weeds) or regrowth from the growing point (broadleaf weeds).
Table 1. Herbicides and rates used for screening various weed species for resistance.
Weed species | Herbicide (note rates are of active ingredient) |
|---|---|
Sowthistle | Glyphosate (729 g ae/ha) |
Fleabane | Glyphosate (729 g ae/ha) † |
Feathertop Rhodes grass | Glyphosate (729 g ae/ha)†* |
Awnless barnyard grass | Glyphosate (729 g ae/ha) |
Windmill grass | Glyphosate (729 g ae/ha) |
†Not registered for control of this weed species
*Glyphosate used as stand-alone and not in tank-mix with 2,4-D amine as per label requirement.
** Used alone as post-emergence . Note that imazapic is only registered for stand-alone use against these weeds as a pre-emergent application.
***As per APVMA permit PER89322 (but without the double knock)
Results
Sowthistle
In total 221 sowthistle populations from Queensland and New South Wales were collected. Only 197 populations were viable for screening with glyphosate and 2,4-D amine. Glyphosate resistance was detected in 14% of the populations tested (Table 2), while 100% of the populations were susceptible to 2,4-D amine. Screening of 136 populations with Velocity® and chlorsulfuron showed that Velocity was able to control all populations while poor control (95% populations survived) was achieved with chlorsulfuron (Table 2).
Table 2. Percentage of populations surviving treatment with different herbicides assessed 21 DAT.
Weed Species | Number of populations tested | Glyphosate (%) | 2,4-D amine (%) | Chlorsulfuron (%) | Velocity (%) |
|---|---|---|---|---|---|
Sowthistle | 197 | 14 | 0 | ||
136 | 95 | 0 | |||
Fleabane | 61 | 100 | 0 | n/a | n/a |
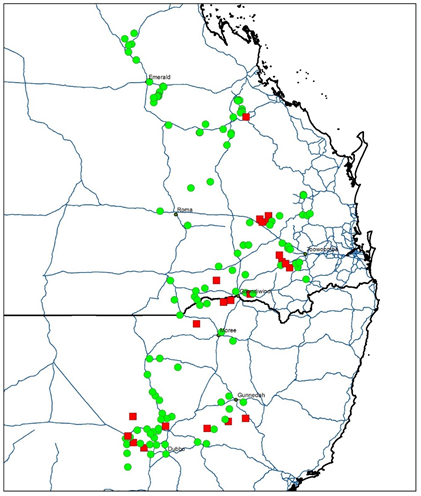
Figure 1. Map of glyphosate resistant and susceptible sowthistle populations across the northern grain cropping region. Red squares represent resistant populations while green circles represent susceptible populations.
Fleabane
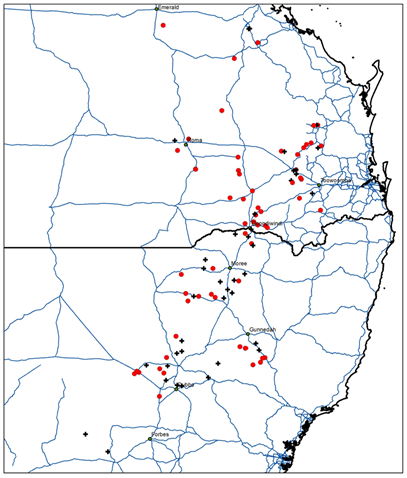
There were 100 fleabane populations collected across the Northern region but only 61 viable populations (Figure 2) were screened with glyphosate and 2, 4-D amine. Glyphosate, which is not registered to control fleabane, failed to control all of the fleabane populations tested. However, no population survived treatment of 2, 4-D amine (Table 2).
Figure 2. Map of fleabane populations across the northern grain cropping region surviving glyphosate application. Red circles are populations surviving the target glyphosate application rate, while black crosses represent non-viable populations.
Feathertop Rhodes grass
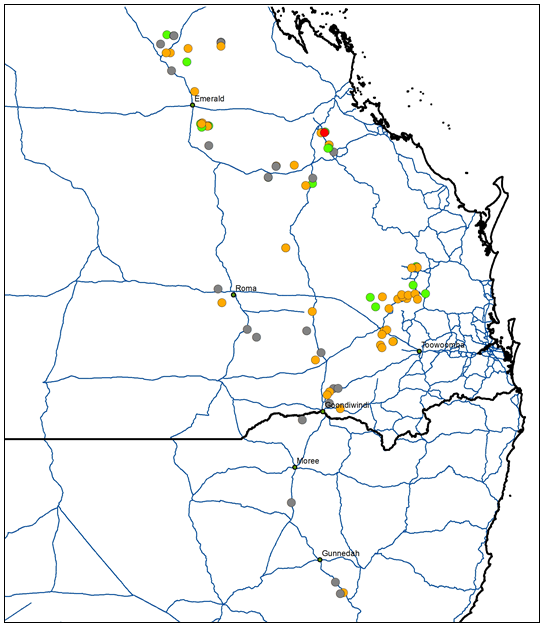
Screening of 62 viable populations revealed 68% survived the glyphosate target rate (noting that glyphosate is not registered for FTR control) (Figure 3, Table 3). One population survived treatment with haloxyfop, while all populations were controlled with clethodim and paraquat.
Table 3. Percentage of feathertop Rhodes grass populations surviving treatment with different herbicides, assessed 21 DAT.
Weed species | Number of populations tested | Glyphosate (%) | Haloxyfop (%) | Clethodim (%) | Paraquat (%) |
|---|---|---|---|---|---|
Feathertop Rhodes grass | 62 | 68 | 2 | 0 | 0 |
Figure 3. Map of glyphosate resistant and susceptible feathertop Rhodes grass populations across the northern grain cropping region. A red circle represents a glyphosate and haloxyfop resistant population, yellow circles represent glyphosate resistant populations that are susceptible to haloxyfop, green circles represent populations susceptible to all herbicides tested, and grey circles represent populations that were not viable.
Awnless barnyard grass
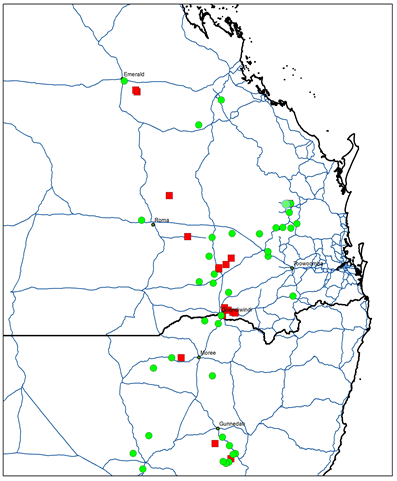
Screening of 42 viable populations revealed 36% were resistant to glyphosate (Figure 4, Table 4), while all of the populations were susceptible to propaquizafop, clethodim and imazapic (noting imazapic was applied as a foliar application and not a soil residual treatment).
Figure 4. Map of glyphosate resistant and susceptible awnless barnyard grass populations across the northern grain cropping region. Red squares represent resistant populations while green circles represent susceptible populations.
Table 4. Percentage of awnless barnyard grass, windmill grass and liverseed grass populations surviving treatment with different herbicides, assessed 21 DAT.
Weed species | Number of populations tested | Glyphosate (%) | Propaquizafop (%) | Clethodim (%) | Imazapic (%)* |
|---|---|---|---|---|---|
Awnless barnyard grass | 42 | 36 | 0 | 0 | 0 |
Liverseed grass | 3 | 0 | 0 | 0 | 0 |
Windmill grass | 12 | 58 | n/a | 0 | n/a |
* Imazapic was applied as a foliar application, and not a soil residual treatment
Windmill grass
Screening of 12 viable populations revealed that more than half of them (58%) survived the target glyphosate rate (Table 4). All of the populations were controlled by clethodim.
Liverseed grass
There were only 14 liverseed grass populations collected across the Northern region. Almost none of them were viable. Herbicide resistance screening of the viable populations (3) showed no evolved resistance to any of the herbicides tested (Table 4).
Discussion
For years, there have been anecdotal reports of certain weed species becoming more difficult to control. This project provides, for the first time, proof of widespread occurrence of herbicide resistance in key weed species in the Northern region.
Glyphosate resistance is the most pressing issue, as all of the weed species tested have populations that were able to survive robust glyphosate application rates, except for liverseed grass (noting only three liverseed grass populations were tested). Especially worrying is fleabane, where total glyphosate failure was recorded. Frequency of glyphosate survival for the other weed species ranges from low (14%, sowthistle) to moderate/severe (68%, feathertop Rhodes grass).
Glyphosate is not registered for control of feathertop Rhodes grass, as it has been known to provide unreliable control. The inclusion of glyphosate screening to feathertop Rhodes grass was to confirm the extent of survival.
Despite the number of weed species and populations that have evolved glyphosate resistance, it should be noted that other herbicides tested were still effective. 2,4-D amine controlled all of the fleabane and sowthistle populations tested, including the ones that survived glyphosate.
Group A herbicides such as haloxyfop, propaquizafop and clethodim gave good control of the summer grass weeds, with only one feathertop Rhodes grass sample surviving haloxyfop treatment.
Imazapic, a group B herbicide that was used post-emergence in our screening, provided good control of awnless barnyard grass and liverseed grass populations. It should be noted imazapic is only registered for pre-emergent control of both awnless barnyard grass and liverseed grass. For post-emergent application the label requires it to be tank-mixed with paraquat. As such, these results should be interpreted with caution.
Viability of the weed seeds was one of the issues in our herbicide resistance screening. All of the weed species tested had populations with non-viable seeds, ranging from 10% (sowthistle, glyphosate and 2, 4-D amine) and up to nearly 80% of populations (liverseed grass). Some of these non-viable seeds were immature, and we believe some of the weed seeds were exposed to herbicide treatment shortly before collection, which could contribute to non-viability of seeds.
The presence of evolved herbicide resistant weeds in the Northern region is expected, given the reports of resistant weeds in the past. However, the extent of the some of the resistance, e.g. fleabane to glyphosate, is truly worrying. This survey offers, for the first time, an overview of the widespread nature of herbicide resistant weeds in the Northern region in key weed species. Continued heavy reliance on herbicides for weed control is likely to exacerbate the resistance challenge for growers and agronomists. Other weed management tactics that incorporate non-chemical weed control such as crop competition, targeted tillage, and harvest weed seed control (Widderick, Ruttledge, McKiernan, personal communication) should be seriously considered to ensure the herbicides that we have now continue to be effective in the future.
References
Adkins, S. W., D. Wills, M. Boersma, S. R. Walker, G. Robinson, R. J. MCLeod and J. P. Einam (1997). "Weeds resistant to chlorsulfuron and atrazine from the north-east grain region of Australia." Weed Research 37(5): 343-349
Cook, T. (2014). The Northern Grains Region: its unique herbicide resistance challenges. Proceedings of the 19th Australasian Weed Conference. Hobart, TAS: Tasmanian Weed Society.
Cook, T., Davidson, B., Miller, R., (2004). "A new glyphosate resistant weed species confirmed for northern New South Wales and the world: common sowthistle (Sonchus oleraceus)." seeds 1000(2).
Widderick, M., Cook, T., McLean, A., Churchett, J., Keenan, M., Miller, R. and Davidson, B. (2014). "Improved management of key northern region weeds: diverse problems, diverse solutions." Proceedings of the 19th Australasian Weed Conference. Hobart, TAS: Tasmanian Weed Society.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, DAF and Charles Sturt University. The authors would like to thank them for their continued support.
Contact details
Dr Michael Widderick
Department of Agriculture and Fisheries
13 Holberton Street, Toowoomba, Queensland 4350
Ph: 07 4529 1325
Email: Michael.Widderick@daf.qld.gov.au
GRDC Project Code: UCS1507-001RTX, UCS1507-001RTX,