Stubble Olympics: the cereal pathogen 10cm sprint
Stubble Olympics: the cereal pathogen 10cm sprint
Author: Toni Petronaitis (NSW DPI, University of New England), Clayton Forknall (DAF QLD), Steven Simpfendorfer (NSW DPI) and David Backhouse (University of New England) | Date: 28 Jul 2020
Take home messages
- Wetter is better (for cereal pathogens): moist conditions promoted growth of pathogenic fungi (Fusarium pseudograminearum, Bipolaris sorokiniana and Pyrenophora tritici-repentis) within post-harvest cereal stubble, meaning inoculum levels of crown rot, common root rot and yellow spot may increase if wet weather is experienced after harvest
- Not all cereal stubble is created equally: some pathogens progressed further in oat than bread wheat stubble. Additionally, there are indications that the resistance ratings of varieties and crops do not reflect the extent of saprophytic growth post-harvest
- Each cereal pathogen had a unique stubble-colonisation pattern: the crown rot fungus was the quickest to progress within all stubble types and the yellow spot pathogen was the slowest. This is likely to influence which pathogen dominates in following seasons if mixed infections have occurred in the same crop
- Reducing cereal stubble biomass may limit the post-harvest progression of pathogenic fungi in stubble, thereby reducing the amount of inoculum carried forward. Options could include selection of low-biomass varieties, low harvest heights or cutting for hay, however field validation is required.
Introduction to post-harvest (saprophytic) growth of cereal pathogens
Fusarium crown rot, yellow leaf (tan) spot and common root rot are significant diseases of cereal crops in Australia with one important thing in common: they are all caused by stubble-borne pathogens. Expanding adoption of conservation agriculture practises (such as cereal stubble-retention) makes these diseases very difficult to control because inoculum is preserved in the previous years’ cereal rows. Surprisingly, we don’t know much about what these pathogens are doing between harvest of an infected crop and sowing of the next susceptible crop (except that they generally persist long enough to cause disease in following seasons).
Reports of pathogen growth (we call it saprophytic growth) in standing cereal stubble has been reported, but it is still unclear how frequently this saprophytic growth occurs, and if it contributes to inoculum build-up or disease risks in future cereal crops. Previously, we showed that the crown rot pathogen Fusarium pseudograminearum can colonise cereal stubble at an average rate of 1cm/day under moist conditions (Petronaitis et al., 2020). So, saprophytic growth can be rapidif moisture is not limiting. But how much moisture do we need for saprophytic growth to start, and is it the same for other pathogenic fungi, like Bipolaris sorokiniana (causative agent of common root rot) and Pyrenophora tritici-repentis (causative agent of yellow leaf spot) in different cereal stubbles?
Knowing how these pathogens behave in post-harvest cereal stubble may be the key to controlling them effectively in conservation agriculture systems. As such, we set up an experiment, named the “Stubble Olympics”, to explore the following questions: (1) what moisture conditions induce saprophytic growth of these different pathogens, (2) how far and fast inoculum may progress under such conditions, and (3) if crop selection (or other management strategies) can be used to supress saprophytic growth.
The “Stubble Olympics” experiment
Who are our contestants in the “Stubble Olympics”? Three important cereal pathogens: two isolates each of Crown rot (F. pseudograminearum), common root rot (B. sorokiniana) and yellow leaf spot) P. tritici-repentis. Each isolate was placed inside individual tillers of cereal stubble from four crop types (Table 1) and tested for saprophytic fitness by measuring their growth under moisture conditions ranging from 90% to 100% relative humidity (RH) in 2.5% RH increments. Two varieties of bread wheat and two varieties of barley were selected to have either a relatively susceptible or relatively resistant disease rating for each pathogen.
Table 1. Cereal stubble collection (location), variety information (species, variety and class), and disease ratings for crown rot (CR), common root rot (CRR) and yellow leaf spot (YLS). Rating information sourced from Winter Crop Variety Sowing Guide 2019 (Matthews and McCaffrey, 2019)
Cereal species | Variety (class) | Crop location | CR rating | CRR rating | YLS rating |
|---|---|---|---|---|---|
Bread Wheat | EGA Gregory (APH) | Narrabri | S | MS-S | S |
LongReach Lancer (APH) | Narrabri | MS-S | S | MR-MS | |
Durum wheat | DBA Lillaroi (ADR) | Tamworth | S-VS | MS-S | MR-MS |
Barley | Compass | Narrabri | S | MS | NA |
Rosalind | Narrabri | MS-S | S | NA | |
Oat | Eurrabie | Narrabri | NA | NA | NA |
Abbreviations: Australian prime hard (APH), Australian Premium Durum (ADR), not applicable (NA),
moderately resistant (MR), moderately susceptible (MS), susceptible (S), very susceptible (VS)
Individual tillers were inoculated at the base with an agar plug of one of six pathogen isolates, and this end of the tiller was inserted onto a metal nail plate to simulate standing stubble. Custom-built humidity chambers (Figure 1) were used to impose the different RH treatments on the inoculated cereal stubble. Humidity chambers were run for 7 days at constant temperature (25˚C) under alternating ultra-violet light (12 h light/12 h dark). Saprophytic growth was measured as the number of tiller segments (1cm length) and position (1-10) which the pathogen was recovered from on agar.
The experiment was repeated twice over time, with treatments arranged according to a split-plot design, where RH treatments were randomised to main plots and crop, variety, pathogen, isolate combinations were randomised to sub-plots.
 Figure 1. Example of controlled humidity chamber design containing “standing” stubble
Figure 1. Example of controlled humidity chamber design containing “standing” stubble
Moisture induces saprophytic growth of pathogens in cereal stubble
Moisture (relative humidity (RH)) had a profound effect on the saprophytic growth of all three of our cereal pathogen contestants (Figure 2). In general, pathogens progressed further (i.e. maximum length of stubble colonised) as RH increased. However, each species responded differently to each moisture scenario, except for the driest treatment (90% RH) where all pathogens experienced little-to-no growth. Once moisture was increased to 92.5% RH and 95% RH, the crown rot pathogen was able to colonise stubble twice as fast as the other two pathogens. The yellow spot and common root rot fungi required moisture levels of 97.5% to progress significantly. All pathogens were able to progress the farthest, and produced the most inoculum, under saturated (100% RH) conditions.
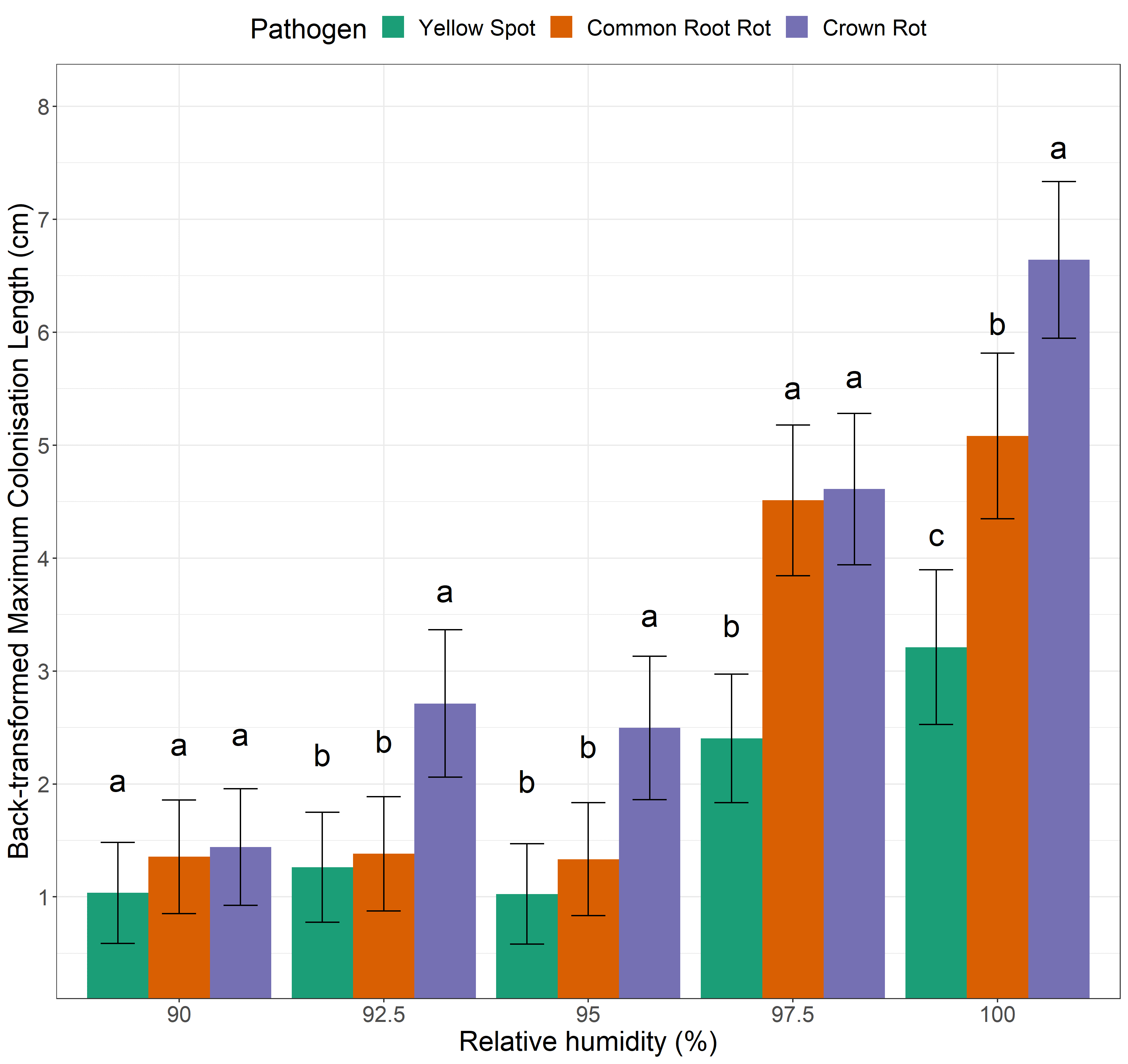 Figure 2. Maximum colonisation (cm) of cereal stubble by three cereal pathogens subject to moisture conditions of 90% RH, 92.5% RH, 95% RH, 97.5% RH or 100% RH for seven days. Note LSD letters only enable comparisons between pathogens within a humidity treatment (not between humidity treatments). Values with the same letter are not significantly different (P=0.05). Error bars represent approximate standard error of the mean.
Figure 2. Maximum colonisation (cm) of cereal stubble by three cereal pathogens subject to moisture conditions of 90% RH, 92.5% RH, 95% RH, 97.5% RH or 100% RH for seven days. Note LSD letters only enable comparisons between pathogens within a humidity treatment (not between humidity treatments). Values with the same letter are not significantly different (P=0.05). Error bars represent approximate standard error of the mean.
Inoculum can progress very fast under moist conditions (which pathogen will take home the gold?)
At 100% RH, the crown rot pathogen experienced significantly faster growth than both other pathogens (~1cm/day – takes home the gold!), whilst the common root rot pathogen was significantly faster (~0.7cm/day – silver!) than the yellow spot pathogen (~0.45cm/day – bronze!) (Figure 2). Multiple-pathogen infections (e.g. crown rot + common root rot) are common in the northern region (Simpfendorfer and McKay, 2019). Our work suggests that under saturated conditions (i.e. rainy, dewy or foggy weather) the crown rot pathogen could rapidly vertically colonise stubble of plants already infected during the growing season, making it more likely to dominate in following seasons.
Selecting crops for resistance won’t help supress growth in the saprophytic phase
Remember how the two varieties each of bread wheat and barley were selected for their difference in disease resistance ratings (Table 1)? Interestingly, no differences in progression (cm) (Figure 2) or colonisation (%) (Figure 3) were observed between the two varieties of the same crop type regardless of the resistance rating. This indicates that varietal resistance does not reduce saprophytic growth (i.e. further inoculum production) post-harvest. Oats, and barley in the case of yellow spot, have no resistance ratings for the selected diseases because they are not typically considered important hosts. However, the yellow spot pathogen has been detected on non-host crops such as barley in the northern region (Simpfendorfer and McKay, 2019). In most cases, the pathogens are not causing disease and are believed to be saprophytically colonising senescing plants late in the season. Therefore, it is quite troubling that the three pathogens produced the same or more inoculum on oat stubble at 100% RH (Figure 3). For example, the yellow spot pathogen produced significantly less inoculum on bread wheat stubble (a recognised host) under moist conditions compared to oat (non-host). It’s possible that the denser tissue and lower biomass of bread wheat stubble may help slow saprophytic colonisation. On the other hand, oat stubble may allow faster progression due to less dense/hollow culms or allow more nutrient exploitation by the fungi (increased lignin content and higher digestibility). So, even if only low levels of infection are experienced during the growing season, or disease is not expressed due to favourable seasonal conditions or plant tolerance, rapid colonisation may still occur after plant senescence.
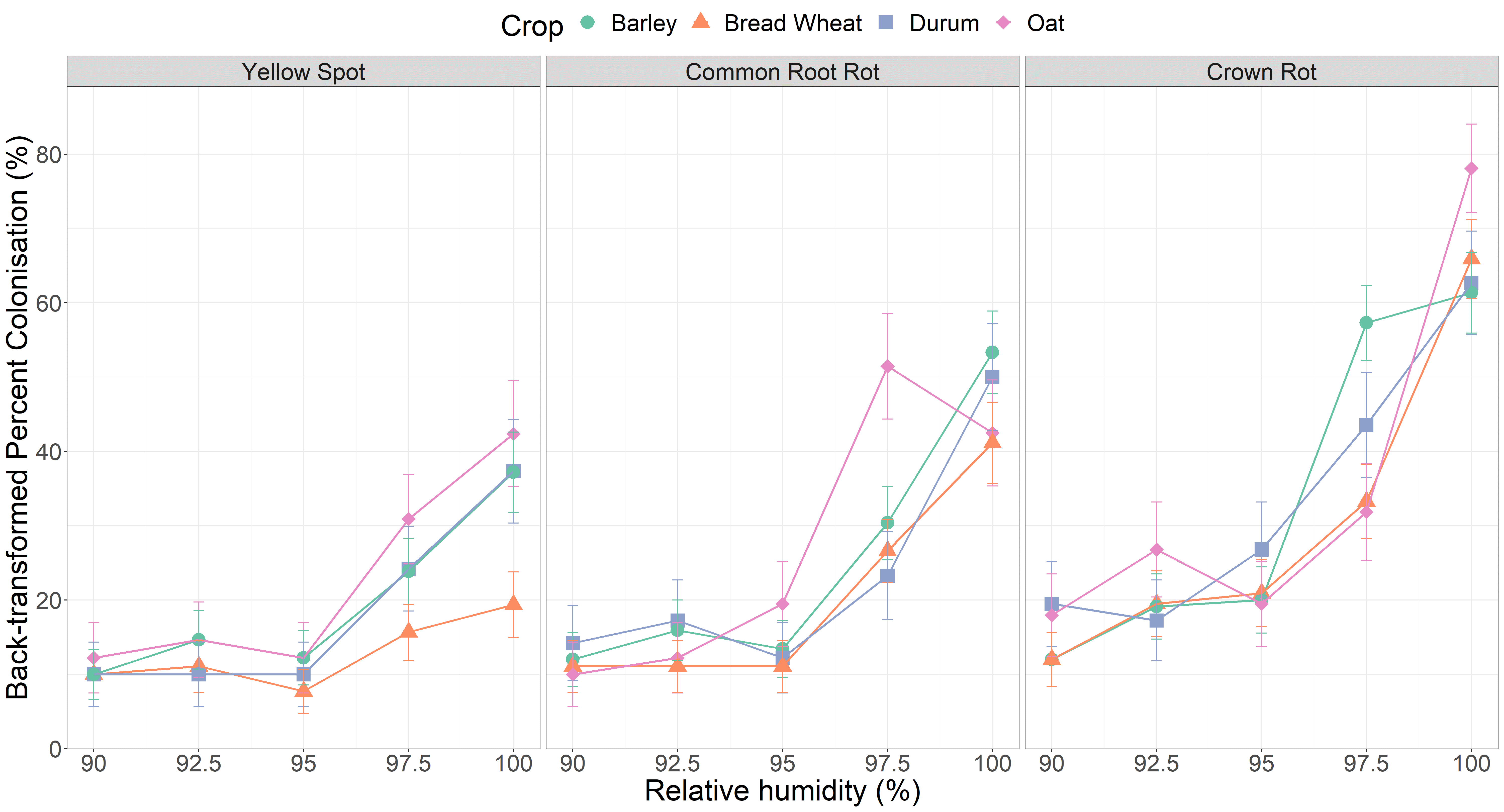 Figure 3. Inoculum production as a percentage (%) of different types of cereal stubble colonised by three pathogens subject to moisture conditions of 90% RH, 92.5% RH, 95% RH, 97.5% RH or 100% RH for seven days. Error bars represent approximate standard error of the mean.
Figure 3. Inoculum production as a percentage (%) of different types of cereal stubble colonised by three pathogens subject to moisture conditions of 90% RH, 92.5% RH, 95% RH, 97.5% RH or 100% RH for seven days. Error bars represent approximate standard error of the mean.
How may this knowledge be important to growers?
Harvest height to manipulate stubble biomass – still a work in progress
Reducing harvest height is a quick way to reduce cereal stubble biomass (Figure 4, field data not shown). This might be useful in severely infected paddocks by removing disease inoculum and/or limiting the amount of vertical stubble available for further saprophytic growth. In severe cases, for example durum wheat affected by crown rot in a dry season, the stubble may already be extensively colonised at harvest (Figure 4). Field testing is underway to reveal if saprophytic growth occurs within taller stubble (i.e. beyond levels at harvest) and whether shorter stubble can limit further growth.
 Figure 4. Harvest-height disease management trial at Narrabri, NSW. Durum wheat was harvested at three heights: 10-15cm (A), ~30cm (B) and 40-45cm (C). Far right: recovery of crown rot pathogen F. pseudograminearum (red-brown colonies) at harvest shows significant colonisation within the stem at harvest (up to 30 cm). Arrows indicate where the pathogen was recovered from along the stubble length.
Figure 4. Harvest-height disease management trial at Narrabri, NSW. Durum wheat was harvested at three heights: 10-15cm (A), ~30cm (B) and 40-45cm (C). Far right: recovery of crown rot pathogen F. pseudograminearum (red-brown colonies) at harvest shows significant colonisation within the stem at harvest (up to 30 cm). Arrows indicate where the pathogen was recovered from along the stubble length.
Modelling saprophytic growth based on weather patterns/predictions – we’re in the early days
Controlling the humidity chambers to be at 25 ᵒC enabled detailed investigation of pathogen response to moisture in stubble. However, modelling saprophytic growth in the field would require knowledge of these growth patterns across a range of temperatures, because air can hold more water at higher temperatures. For instance, during hotter months there is more total water in the air, but we don’t know if this water is available to stubble-borne pathogens until high RH (close to dew point) is achieved. Air gives up moisture more freely at lower temperatures (dew point is lower), hence we generally experience more dewy/frosty or foggy mornings during winter. Determining whether the pathogens respond to total water or dew point (or both) will be essential for modelling saprophytic growth.
Should growers be concerned about saprophytic growth of pathogens in cereal stubble?
The short answer: be alert, not alarmed. Right now, we are still trying to understand if and how saprophytic growth of cereal pathogens during fallow and non-host rotation may affect disease risk in subsequent seasons. It is possible that the recent higher rainfall experienced in many areas may have spiked pathogen levels right before sowing, placing new crops at a higher risk than in previous (drier) years. Furthermore, the extended dry conditions (2017-19 seasons) have allowed inoculum to persist at damaging levels for much longer than normal (2-4 seasons). So, be vigilant about checking this years’ cereal crops for disease symptoms and consider appropriate in-crop management strategies if necessary.
Always remember that seasonal conditions can affect cereal stubble biota (the good and the bad) during fallows and non-host rotations, and stubble is not “dormant” during these times. In summary:
- Dry conditions allow inoculum (and cereal stubble) to persist longer (2-4 years). Stubble will not be as accessible to beneficial microbes (with higher moisture requirements) which can suppress pathogens. Our work reinforces how Fusarium species are especially suited to survive and grow in drier conditions.
- Wet conditions, like those applied in our study, can potentially increase inoculum, but the cereal stubble will also decompose faster if prolonged wet weather is experienced. Moisture may increase the activity of beneficial microbes, helping with stubble decomposition and pathogen suppression. Moist conditions also promote spore production by these pathogens, and these can persist in soil for many years in the absence of stubble (e.g. conidia of common root rot pathogen).
Testing using PREDICTA® B is a very effective method for determining disease risk (following the up-to-date protocol of adding cereal stubble to the sample). If your paddock/s have returned a below detection limit or low risk PREDICTA B test for cereal disease, then you can continue following best practise agronomy for your next cereal crop.
References
Matthews, P., and McCaffrey, D. (2019). Winter crop variety sowing guide. NSW Department of Primary Industries, 20-65.
Petronaitis, T., Forknall, C., and Simpfendorfer, S. (2020). Crown rot stubble inoculum levels within season and further growth after harvest. Northern NSW research results 2019, 87-91. NSW Department of Primary Industries, 87-91.
Simpfendorfer, S., and McKay, A. (2019). What pathogens were detected in central and northern cereal crops in 2018? GRDC Update, Goondiwindi, 106-115.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC and the authors would like to thank them for their continued support. Ms Petronaitis would like to thank the GRDC and NSW DPI for co-funding her GAPP PhD scholarship (BLG211/304) and Associate Professor David Backhouse (UNE), Dr Steven Simpfendorfer (NSW DPI) and Dr Graham Brodie (UniMelb) for their PhD supervision. Rick Graham and Gururaj Kadkol (NSW DPI) are thanked for providing cereal stubble for the Stubble Olympics experiment. Technical assistance provided by Chrystal Fensbo, Finn Fensbo, Jason McCulloch, Stephen Morphett, Michael Dal Santo and Jim Perfrement is gratefully acknowledged.
Contact details
Toni Petronaitis
NSW DPI
4 Marsden Park Road, Tamworth NSW 2340
Ph. 02 6763 1219
Email: toni.petronaitis@dpi.nsw.gov.au
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
® Registered trademark
