Aphid and insecticide resistance management in grain crops
Author: Marielle Babineau, Anthony Van Rooyen, James Maino, Jenny Reidy-Crofts, Owain Edwards, Paul Umina | Date: 21 May 2020
Take home messages
- A high number of green peach aphid populations are resistant to pyrethroids, carbamates, organophosphates, and neonicotinoids.
- Multiple green peach aphid populations from a specific region showed a temporary sensitivity shift to sulfoximines (sulfoxaflor, Transform®) due to an unknown mechanism.
- Significant differences in pyrethroid sensitivity were found between populations of oat aphid.
- The green peach aphid insecticide resistance management strategy has been updated and should be consulted by growers and advisers.
- Rotate available insecticide modes of action (new modes of action to be registered in coming years).
- Controlling green bridges around paddock and sowing into standing stubble is recommended.
- Proper identification of aphid species is necessary to assess risk of resistance and choice of insecticide to be used.
- Monitoring of aphid predators and parasitoids allows chemical-free control of insecticide resistant aphids.
Background
Aphids are major crop pests throughout the world, attacking a broad range of crops through direct feeding, and as a vector of viral diseases. In Australia, the cost of aphid injury has been estimated at $200-$400 million per year. The green peach aphid (Myzus persicae, GPA) has evolved resistance to a wide range of commonly used insecticides. There is a need to develop resistance management strategies that minimise the risk of increased resistance evolution, focus on targeted use of pesticides and include non-chemical options.
In Australia, the oat aphid (Rhopalosiphum padi) causes feeding injury which can reduce cereals yield by 6%, with the damage caused by aphid-vectored viruses reducing yield of cereal crops by up to 30%. Aphid control in these crops is achieved almost exclusively with chemical insecticides, and there is growing concern about insecticide resistance evolution in multiple aphid species.
Method
This project documented the frequency and spread of insecticide resistance in GPA through a national insecticide resistance surveillance program throughout Australia. Over 400 GPA populations were screened for resistance to carbamates, organophosphates, synthetic pyrethroids, neonicotinoids and sulfoximines. Screening was completed using optimised phenotypic methodologies, newly developed molecular markers for GPA populations and testing over multiple known genetic resistance mechanisms. The assessment of resistance was based on genetic screening of alleles for MACE, kdr, super-kdr, as well as amplification of the E4 esterase gene, and finally the CYP6CY3 (P450) copy number, which confer resistance to carbamates, pyrethroids, organophosphates, and neonicotinoids. The risk of incursion and local evolution of the sulfoximine and neonicotinoid-resistant mutation (known as “R81T”) has been evaluated and investigated through predictive modelling and a large network of field traps, followed by genetic testing for this mutation.
An extensive review of resistance risk in aphid species other than GPA was also completed. New knowledge was developed regarding the risk of resistance evolution in grain aphids and based on risk profiles and considerable base-line sensitivity data was established for multiple insecticides for two species deemed to be at risk of resistance evolution in Australia; the cowpea aphid and oat aphid. Nine field populations of oat aphid were collected from localities representing the major grain growing regions of Australia; Tamworth (TMW), Picola (PIC), Dookie (DOK), Pimpinio (PIM), Back Valley (BKV), Adelaide (ADE), Manjimup (MNJ), Capel (CAP) and Katanning (KAT). Toxicity data for each population was established against four insecticide classes (organophosphates, synthetic pyrethroids, carbamates, neonicotinoids).
Results and discussion
Green peach aphid resistance monitoring
Insecticide resistance maps show that resistance is commonplace; almost all (>90%) populations tested showed resistance to carbamates, pyrethroids, organophosphates and neonicotinoids (Figure 1).
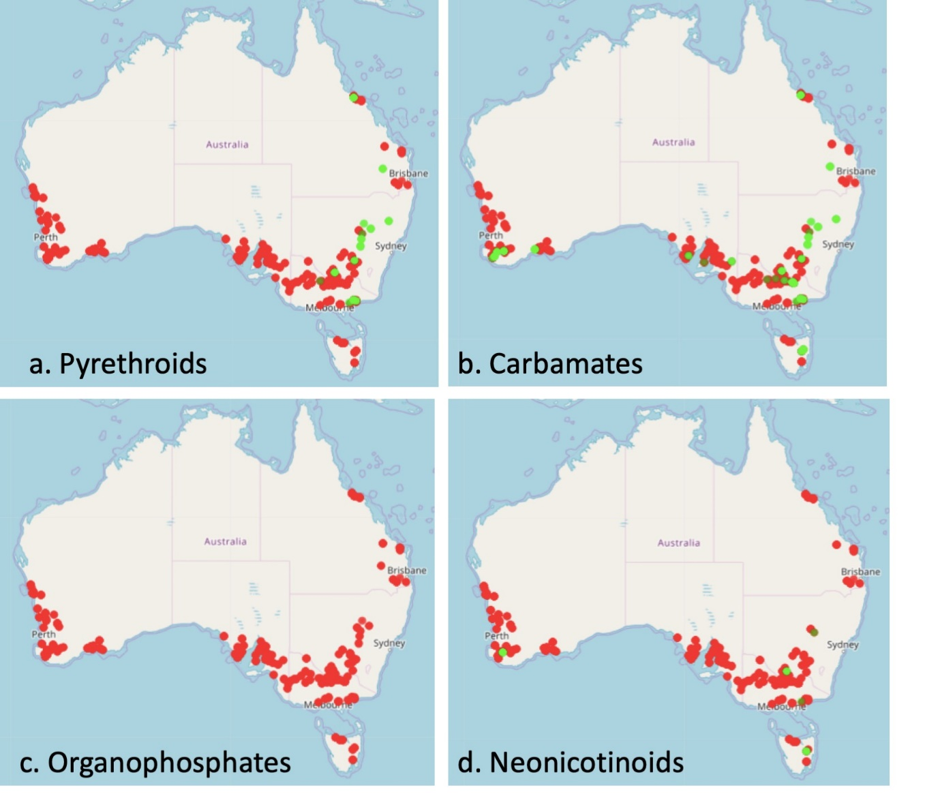
Figure 1. Insecticide resistance maps of green peach aphid collected during 2017/2018 for pyrethroids, carbamates, organophosphates, and neonicotinoids. Red dots represent resistant population location, green represent susceptible population location.
Genotyping has revealed GPA from many field-collected populations are resistant clones, possessing resistance to organophosphates, carbamates, pyrethroids and neonicotinoids. The use of pyrethroids and pirimicarb (even at high rates) will not provide control against these populations. Interpreting resistance-testing results for organophosphates is more complex. The amplified carboxyl-esterase mechanism leads to organophosphate resistance in GPA. This mechanism is unusual because it is regulated by DNA methylation and can be ‘switched on’ in response to environmental conditions such as weather events or pesticide exposure. As a result, aphids carrying the E4/FE4 gene amplification can quickly adapt to survive organophosphates. The GPA populations that have this gene amplification are expected to have a moderate level of resistance (5- to 20-fold) to organophosphates; including dimethoate, omethoate and chlorpyrifos. A baseline sensitivity of spirotetramat and cyantraniliprole has demonstrated high sensitivity in all GPA populations tested (de Little et al. 2017a).
For neonicotinoids, a subset of populations was screened using phenotypic laboratory bioassays as well as testing for the overexpression of the P450 monooxygenase CYP6CY3 detoxifying gene. Low-level resistance was found across most field-collected populations. Under field conditions, this resistance mechanism is likely (although not confirmed) to shorten the length of protection offered by neonicotinoid-based seed treatments on canola. Importantly however, complete chemical field failures are unlikely given the low level of resistance exerted by the overexpression of the CYP6CY3 gene (de Little et al. 2017b).
A sensitivity shift was also identified to sulfoximines in a small number of GPA populations collected from Western Australia (WA) around the Esperance region. Four populations had significantly higher tolerance to sulfoxaflor, based on LC50, compared to other populations and the susceptible control (Figure 2). The resistance factor was 7, 8, 10, 23 for Cascade1, Cascade2, Munglinup1, and Munglinup2, respectively. These represent tolerance to dosage below the field rate.
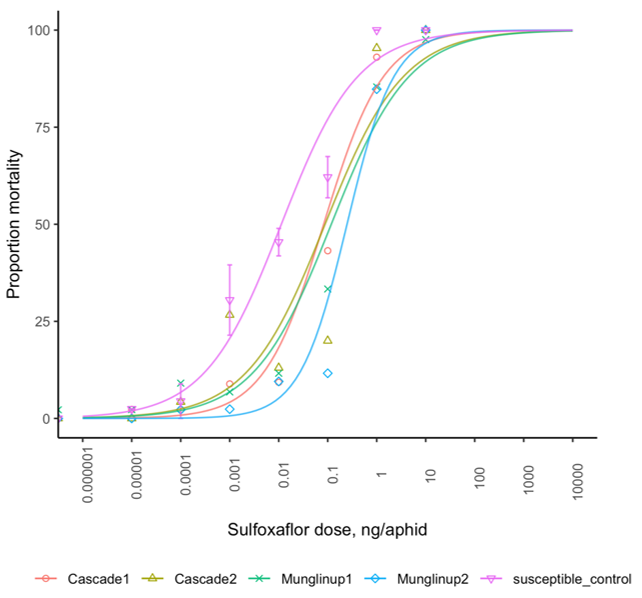
Figure 2. Dose response of sulfoxaflor for four GPA populations and the susceptible control.
Within nine to eleven months of the original phenotypic assay, Munglinup2 showed a significant decrease in sulfoxaflor tolerance, while Cascade1, Cascade2 and Munglinup1 remained at the same sensitivity level as before (Table 1). Recently, Wang et al. (2018) selected a strain of M. persicae for resistance to sulfoxaflor in the laboratory, which showed 199-fold resistance after 45 generations compared with the starting population. The mechanism responsible for this laboratory-selected resistance has not yet been elucidated. Interestingly, Wang et al. (2018) found a significant decrease in aphid survival rate, longevity, fecundity and duration of production compared with the susceptible strain, suggesting strong fitness penalties. Perhaps this, at least partly, explains the results observed for Munglinup2.
Table 1. Sulfoxaflor resistance ratio (RR) over time for four GPA populations collected from the Esperance region in 2018.
Initial Resistance ratio (RR) | 3-4 months later RR | 9-11 months later RR | |
|---|---|---|---|
Cascade1 | 7 | 7 | 7 |
Cascade2 | 8 | 4 | 5 |
Munglinup1 | 10 | 6 | 6 |
Munglinup2 | 23 | 6 | 0 |
None of the known mechanisms of resistance to other modes of action explained the sulfoxaflor sensitivity shift observed. Overseas, the mutation R81T, present in the loop D region of the b-1 subunit of the nicotine acetylcholine receptor gene, is present in European GPA populations and results in sulfoxaflor resistance (observed resistance ratio of approximately 70). This mutation was screened in the above populations and was not observed.
To understand the domestic risk of evolution of further neonicotinoid resistance, such as R81T mutation, the spatial patterns in neonicotinoid applications in Australia was quantified by combining land use data with sales and market research data contributed by agrichemical and agribusiness companies (Cushen et al., submitted). In line with global trends, nearly half the volume of agricultural neonicotinoid products in Australia are applied as seed treatments in cereal, oilseed, cotton and legume crops. This poses risks for the local evolution of R81T mutation in Australia. Dryland cropping regions of cereals, oilseed, and legumes in south-eastern Australia with high estimated usage of neonicotinoids were frequently identified near stone fruit orchards, which has implications for sexual reproduction, thus evolutionary response, in GPA. Similar conditions exist in the south-west of Western Australia, although with lower estimated usage intensity of neonicotinoid products.
The R81T mutation was not found in any Australian GPA (Table 2). The consequences of R81T would be significant to the grains industry; rendering all neonicotinoids ineffective, as well as conferring cross-resistance to sulfoximines. Of the 299 yellow sticky traps that were processed across most of the Australian grain growing regions, GPA were detected on 181 or 60% of traps (Table 2). Most GPA detections occurred in southern Australia. Of the 181 GPA positive samples, all were negative for the R81T mutation, supporting the notion that Australia is presently free of this resistant biotype (Maino et al. in preparation).
Table 2. Total number of traps deployed per state, with the number of GPA carrying the R81T mutation (resistant) or not carrying the R81T mutation (susceptible).
STATE | NO GPA | GPA SUSCEPTIBLE | GPA RESISTANT | TOTAL TRAPS |
|---|---|---|---|---|
New South Wales | 18 | 21 | 0 | 39 |
Queensland | 9 | 0 | 0 | 9 |
South Australia | 25 | 19 | 0 | 44 |
Victoria | 54 | 29 | 0 | 83 |
Western Australia | 12 | 112 | 0 | 124 |
TOTAL | 118 | 181 | 0 | 299 |
Variations in oat aphid baseline sensitivity
For three of the chemical classes tested (organophosphates, carbamates and neonicotinoids), there were no differences in the nine oat aphid population responses. However, for pyrethroids, a widely used insecticide class in Australia, there are statistically significant differences between several aphid populations (Figure 3). After 48hrs, there was a significant difference in dose response curves between populations (c2 = 23.92, df = 8, p = 0.002) for alpha-cypermethrin (Umina et al. submitted). The population from KAT responded differently compared to aphids from TMW (p = 0.006), MNJ (p = 0.03) and PIC (p = 0.02). Aphids from these three populations were less sensitive to alpha-cypermethrin compared with KAT (Figure 3). The LC50 values across all nine populations ranged from 0.6 to 24.57mg a.i./L. The field rate of alpha-cypermethrin (125 mg a.i./L) controlled 98%, 93% and 90% of aphids from ADE, MNJ and TMW, respectively. For the remaining six populations, the field rate was adequate in supressing 100% of individuals after 48 hrs.
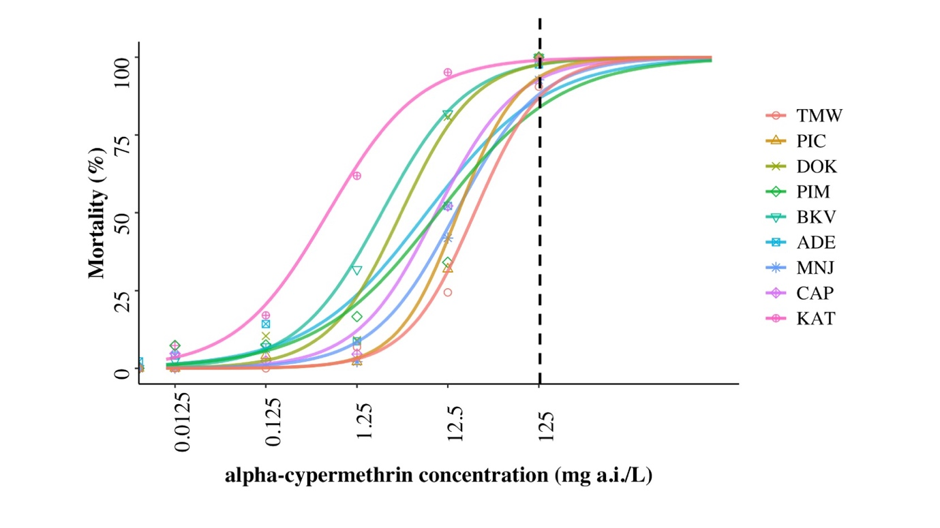
Figure 3. Dose response curves after exposure to alpha-cypermethrin for 48hrs for oat aphid populations collected from Tamworth (TMW), Picola (PIC), Dookie (DOK), Pimpinio (PIM), Back Valley (BKV), Adelaide (ADE), Manjimup (MNJ), Capel (CAP) and Katanning (KAT). Dotted line represents the field rate.
Conclusion and implications for crop aphid management
The research presented above leads to key implications for aphid management.
- Aphid species identification: Each aphid species will show different levels of resistance (or sensitivity) to different insecticides; for example, GPA have widespread resistance to organophosphate, neonicotinoids, carbamates and pyrethroids, while oat aphid have lower sensitivity to pyrethroids. Therefore, the proper identification of aphid species in a paddock is crucial to determine the risk of resistance, and therefore, the selection of management strategies. This key step helps to optimise inputs (financial, labour, time) for efficient management.
- When dealing with GPA, always follow the GPA Insecticides Resistance Management Strategy (IRMS). The research outcomes from this project have informed the update of the GPA IRMS available at the GRDC website www.grdc.com.au/GPAResistanceStrategy. This strategy outlines the regionally relevant management strategies for dealing with GPA .
- Rotating insecticides’ modes of action. This recommendation applies especially to GPA, which has been demonstrated to have a high propensity to evolve insecticide resistance to multiple chemical classes.
- Consult if reduced insecticide efficacy on aphids is suspected. Growers should report and consult their advisers if experiencing insecticide efficacy reduction or failure and/or suspect insecticide resistance. More targeted strategies can be deployed depending on the aphid species and crop type/development stage.
- Control aphids in green bridge and sow into standing stubble (when possible). Aphids can remain in the surrounding area of a recently harvested paddock and this population may serve as a reservoir to infest newly sown crops. In the case of insecticide resistant GPA, it is key to eliminate them from the area. Sowing into standing stubble is a method that reduces the visual cues from migrating aphids and reduces infestation. Reducing the total numbers of aphids in a field minimises the risk of early virus infection.
- Monitor activity of aphid predators and parasitoids. The multiple resistance observed and the high number of GPA populations with a resistant phenotype found in this project compromises chemical management options. There are several aphid predators and aphid parasitoids that can effectively control GPA. These natural enemies will often move in when the GPA population numbers are higher, and therefore, there is a short lag between high GPA numbers and increased activity of natural enemies. Natural enemies can be identified using the GRDC back pocket guide (www.grdc.com.au/BPG-BeneficialInsects-SW). Additionally, insecticides that are less toxic to beneficial insects can be found in the GRDC Insecticide Resistance guide (https://grdc.com.au/insecticide-resistance-in-the-southern-region).
Acknowledgements
The research undertaken in this project was made possible through the financial contributions of GRDC. We thank Corteva Agriscience, CropLife Australia and Bayer Cropsciences for their support. Thanks also thank WA DPIRD, and the many growers and advisors for their assistance with aphid collections.
Useful resources
- PestFacts south-eastern
- Resistance Management Strategy for the Green Peach Aphid in Australia Grains
- Crop Aphids Back Pocket Guide
- Beneficial Insects - The Back Pocket Guide (Southern and Western Regions)
- Insecticide resistance in the southern region: current status, future risk and best management practices
References
Clouston, A.l, Edwards, O, and Umina, P.A. An Insecticide Baseline Study of Australian Broadacre Aphids. Crop and Pasture Science 67, no. 2 (2016): 236. https://doi.org/10.1071/CP15208.
Cushen, A, Umina, P.A, and Maino, J.L. Spatial variation in neonicotinoid usage and priorities for resistance monitoring in Australia. Submitted to Pest Management Science.
Little, S.C de, Edwards, O, Van Rooyen, A, Weeks, A, and Umina, P.A. Discovery of Metabolic Resistance to Neonicotinoids in Green Peach Aphids (Myzus persicae) in Australia. Pest Management Science 73, no. 8 (August 2017b): 1611–17. https://doi.org/10.1002/ps.4495.
Little, S.C. de, and Umina, P.A. Susceptibility of Australian Myzus persicae (Hemiptera: Aphididae) to Three Recently Registered Insecticides: Spirotetramat, Cyantraniliprole, and Sulfoxaflor. Journal of Economic Entomology 110, no. 4 (August 2017a): 1764–69. https://doi.org/10.1093/jee/tox132.
Maino, J.L, Van Rooyen, A, Umina, P.A. Biotype biosecurity – national surveillance for an exotic pesticide-resistant strain of green peach aphid. In preparation.
Umina, P.A, McDonald, G, Maino, J.L, Edwards, O, and Hoffmann, A.A. Escalating Insecticide Resistance in Australian Grain Pests: Contributing Factors, Industry Trends and Management Opportunities. Pest Management Science 75, no. 6 (June 2019): 1494–1506. https://doi.org/10.1002/ps.5285.
Umina, Paul A, Reidy-Crofts, J, Babineau, M, Maino, J, and Edwards, O. Susceptibility of the bird cherry-oat aphid, Rhopalosiphum padi (Hemiptera: Aphidae), to four insecticides. Submitted to Austral Entomology.
Wang Z, Gong, Y, Chen J, et al. 2018. Laboratory selection for resistance to sulfoxaflor and fitness costs in the green peach aphid Myzus persicae. Journal of Asia-Pacific Entomology. 21: 408-412.
Contact details
Dr Marielle Babineau
cesar Pty Ltd, 293 Royal Parade, Parkville, Vic
0393494723
mbabineau@cesaraustralia.com
http://cesaraustralia.com
@cesaraustralia
bit.ly/cesar-youtube
GRDC Project Code: CES00003 (2016-2019), CES2001-001RTX (2020-2023),
Was this page helpful?
YOUR FEEDBACK
