Amelioration of hostile subsoils via incorporation of organic and inorganic amendments and subsequent changes in soil properties, crop water use and improved yield, in a medium rainfall zone of south-eastern Australia
Take home messages
- Deep placement of organic and inorganic amendments increased grain yield in the order of 20 to 50% for four successive years on an alkaline dispersive subsoil at Rand
- Deep placement of organic and inorganic amendments increased root growth, and crop water use from the deeper clay layers during the critical reproductive stages of crop development
- Improvements in grain yield with deep placement of organic and inorganic amendments were associated with a reduction in subsoil pH and improvement in soil aggregation
- Genotypic variability in grain yield response of wheat cultivars grown on alkaline dispersive subsoils has identified varieties and associated traits for enhanced performance and future breeding.
Background
Sodicity, salinity and acidity are significant surface and subsoil constraints that reduce crop productivity throughout the cropping regions of Australia (Sale et al., 2021). The majority of cropping soils contain at minimum one, but more multiple constraints (McDonald et al., 2013). The economic impact to Australian agriculture, expressed by the ‘yield gap’ between actual and potential yield, attributable to subsoil constraints was estimated to be more than A$1.3 billion annually by Rengasamy (2002), and as much A$2.8 billion by Hajkowicz and Young (2005). Of the ‘three’, sodicity is thought to be the most detrimental to productivity, resulting in the greatest yield gap. In Australian wheat-cropping regions alone, this ‘gap’ was estimated to be worth A$1.3 billion per annum in lost income (Orton et al., 2018), while close to 20% of Australia’s land area is thought to be sodic.
Sodic soils, which are characterised by an excess of sodium (Na+) ions, and classified as those with an exchangeable sodium percentage (ESP) greater than 6% (Northcote and Skene, 1972), are often poorly structured, have a high clay content, high bulk density, and are dispersive. These factors result in poor subsoil structure that can impede drainage, promote waterlogging (low water infiltration), and increase de-nitrification (nutrient imbalance), and soil strength (Orton et al., 2018). These properties also impede the infiltration of water into and within the soil, reduce water and nutrient storage capacity, and ultimately the plant available water (PAW) content of the soil. Subsequently, root growth and rooting depth are impeded, as is crop ability to access and extract deeper stored water and nutrients (Passioura and Angus, 2010). Thisis particularly problematic in environments characterised by a dry spring, where the reproductive phase often coincides with periods of water stress, and when the conversion of water to grain has the greatest effect both on yield (Kirkegaard et al., 2007), and the likelihood and magnitude of a yield gap (Adcock et al., 2007).
In southern NSW, winter crops commonly have sufficient water supply either from stored soil water or rainfall during the early growth stages. However, the reproductive phase is often affected by water stress or terminal drought and this is thought to be the major cause of variable grain yield (Farooq et al., 2014). The effect of water stress in the reproductive phase is further impacted by shallow root depth induced by subsoil sodicity. Under such conditions, a key to improving crop productivity is to improve root growth in and through sodic subsoils to enable use of deep subsoil water later in the growing season. Water use at this late stage has a 2 to 3 fold greater conversion efficiency into grain yield (Kirkegaard et al., 2007) than seasonal average based conversions efficiencies (e.g. 20 – 25 kg/mm verses 50 – 60 kg/mm).
While there are large advantages to be gained by improving the soil environment of sodic subsoils, the various amelioration approaches (deep ripping, subsoil manuring, applying gypsum, improved nutrition and use of ‘primer-crops’) have produced variable results (Adcock et al., 2007; Gill et al., 2008). Furthermore the use of subsoil organic material is also impacted by limited local availability, the high cost of suitable organic ameliorants delivered in-paddock, the sometimes large quantities required, the lack of suitable commercial-scale machinery and the poor predictability of when and where the amelioration will benefit crop productivity (Gill et al., 2008; Sale et al., 2019).
Gypsum application has been the most widespread traditional approach used to correct subsoil sodicity. However, problems have included; surface application when the problem is evident in the subsoil, the large quantities of gypsum required to displace significant amounts of sodium and the somewhat low solubility of gypsum.
Genetic improvement is also frequently advocated as an avenue for improving crop productivity and adaptation under different hostile soil conditions (McDonald et al., 2012; Nuttall et al., 2010). Little is known about genetic variation for tolerance of subsoil constraints and how they relate to different plant traits such as elemental toxicity tolerance, canopy cover, rooting depth and harvest index and the integration of these factors in yield response of different genotypes. This limited knowledge is also due to the practical difficulties in measuring dynamic and variable soil constraints under field conditions.
This paper reports on the performance of a barley-wheat-canola-wheat rotation on a Sodosol (Isbell, 2002) soil at a site in the Riverina town of Rand in southern New South Wales in the four years immediately following incorporation of a range of subsoil amendments, and the residual effects of ‘subsoil manuring’ on crop performance, soil physical properties, and access to PAW stored in the soil profile over subsequent seasons. A range of treatments comprising deep-ripping and subsoil incorporation of organic and inorganic amendments at a depth of 20–40cm were compared to, and contrasted with, surface applications, ripping-only and untreated controls. Amendments that could be easily procured or produced as part of a farming system were used in the trial. It is hypothesised that subsoil incorporation of organic or inorganic amendments will provide significant improvements in grain yield, which are associated with changes in the physical properties of the subsoil that result in improved root growth, and access to, and use of, deep soil water.
Method
Rand amendment site
The trial site was located at Rand, in the Riverina region of southern New South Wales in a paddock that had been under a continuous cropping (cereal-canola) for more than 50 years. The soil was a Sodosol with a texture-contrast profile increasing in clay content at depth, and with physical and chemical properties (Table 1.) unfavourable for root growth, including a high bulk density and low hydraulic conductivity.
Table 1. Chemical and physical properties of the soils at different depths at the trial site.
Depth (cm) | pH(H20) | EC (1:5) | Nitrate N (mg/kg) | Exchangeable cations (cmol/kg) | Exchangeable sodium percentage (%) | Bulk density (g/cm3) | Volumetric water content (qν) |
|---|---|---|---|---|---|---|---|
0–10 | 6.6 | 132.1 | 20.6 | 16.1 | 3.8 | 1.40 | 0.120 |
10–20 | 7.8 | 104.0 | 5.8 | 22.6 | 7.3 | 1.52 | 0.163 |
20–40 | 9.0 | 201.5 | 4.1 | 26.7 | 12.5 | 1.50 | 0.196 |
40–50 | 9.4 | 300.5 | 3.0 | 27.5 | 18.1 | 1.48 | 0.232 |
50–60 | 9.5 | 401.3 | 3.0 | 28.8 | 21.8 | 1.53 | 0.237 |
60–100 | 9.4 | 645.0 | 2.9 | 29.7 | 26.4 | 1.55 | 0.218 |
The trial was established in February 2017 as a randomized complete block with 13 treatments (Table 2) and four replicates. Experimental plots were arranged in two blocks (ranges) of 26 plots, separated by a 36m cropped buffer. Individual plots within each block were 2.5m wide (South-North) ´ 20m long (East-West), separated on their long sides by 2m buffers of uncultivated ground. Plots were ripped to a depth of 40cm, and amendments incorporated into the soil via a custom built 3-D ripping machine (NSW DPI), comprising a “Jack” GM77-04 5-tyne ripper (Grizzly Engineering Pty Ltd, Swan Hill, VIC, Australia), configured to 500mm tyne spacings, and topped with a custom designed frame supporting two purpose built discharge hoppers (bins) and a 300L liquid cartage tank. The larger, ~1.6 cubic meter-capacity hopper was designed to deliver organic materials, and can accommodate approximately 1000 kg of material, roughly equivalent to a standard ‘spout top, spout bottom’ bulk bag. The organic amendments were obtained in pellet form for ease of application and consisted of dried pea straw pellets (1.13% N, 0.05% P, 1.34% K; extrusion diam. 7–10mm, length 6–35mm), wheat stubble pellets (0.34% N, 0.15% P, 1.59% K; diam. 7–10mm, length 6–35mm), and dried poultry manure pellets marketed as Dynamic Lifter® (3% N, 2% P, 1.7% K; diam. 7–10mm, length 6–35mm). The amendments were applied three months prior to sowing the first season.
In 2017, experimental plots were sown to Barley (cv. LaTrobe) on the 11th of May at a seeding rate of 70 kg/ha (target plant density 100 plants/m2). Monoammonium phosphate (MAP) was applied at 80 kg/ha as a starter fertiliser at sowing. The crop was sown after spraying with Boxer Gold® (800 g/L prosulfocarb + 120 g/L S-metolachlor), Spray.Seed® (135 g/L paraquat dichloride + 115 g/L diquat dibromide) and Treflan® (480 g/L trifluralin). The crop was harvested on the 21st of November.
In 2018, Wheat (cv. Lancer) was sown on the 15th of May at a seeding rate of 80 kg/ha (target plant density 150 plants/m2). MAP was applied at 80 kg/ha as a starter fertiliser at the time of sowing. The crop was sown after spraying with Spray.Seed, Sakura® (850 g/kg pyroxasulfone), Logran® (750 g/kg triasulfuron) and Treflan. Urea (46% N) at 110 kg/ha (50.6 kg/ha N) was applied at 106 DAS. The crop was harvested on the 6th of December.
In 2019, Canola (Pioneer® 45Y92CL) was sown on the 10th of April at a seeding rate of 4.4kg/ha (target plant density 40 plants/m2). MAP was applied at 90 kg/ha (9 kg/ha N, 19.8 kg/ha P) as a starter fertiliser at the time of sowing. The crop was sown after spraying with a knockdown mixture of herbicides. Urea at 220 kg/ha (101.2 kg/ha N) was applied as a top-dressing at 119 DAS, and Prosaro® (210 g/L prothioconazole + 210 g/L tebuconazole) at 50% bloom as a preventative for Sclerotinia stem rot (132 DAS). The crop was harvested on the 30th of October.
In 2020, wheat (cv. Scepter) was sown on the 16th of May at a seeding rate of 63 kg/ha (target plant density of 120 plants/m2). Diammonium phosphate (DAP) was applied at 78 kg/ha as a starter fertiliser at the time of sowing. The crop was sown after spraying with Spray.Seed, Roundup, Sakura and Treflan. Urea at 150 kg/ha (69 kg/ha N) was applied as a top-dressing 7 DAS prior to rain. The crop was harvested on the 7th of December.
The long-term average annual rainfall at the site is 553mm with a reasonably uniform average monthly rainfall. In 2017, in-season rainfall (April-November) totalled 329mm, while 244mm and 242mm, respectively, were recorded for the same period in 2018 and 2019. Rainfall in both 2018 and 2019 was approximately 25% less than that recorded for 2017, and approximately 65% of the long-term average seasonal rainfall. The long-term average monthly rainfall, and average monthly maximum and minimum temperatures, daily (bars) rainfall events and monthly rainfall at the Rand experimental site for the period 2017-2020 (Figure 1).
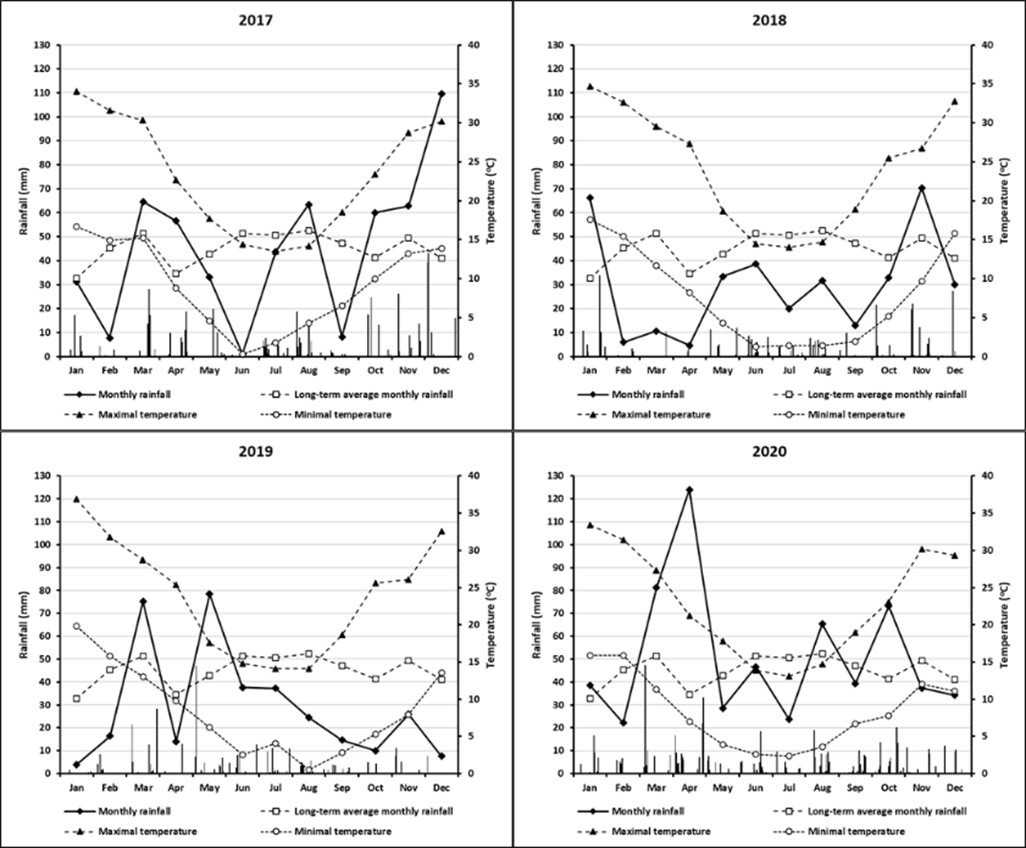 Figure 1. Long-term average monthly rainfall, and average monthly maximum and minimum temperatures, daily (bars) rainfall events and monthly rainfall at the Rand experimental site located at Urangeline East, NSW.
Figure 1. Long-term average monthly rainfall, and average monthly maximum and minimum temperatures, daily (bars) rainfall events and monthly rainfall at the Rand experimental site located at Urangeline East, NSW.
Table 2. Description of the treatments and organic and inorganic amendments used in the trial.
Treatment | Description | Amount of amendment added |
|---|---|---|
1 | Control | Direct sowing |
2 | Deep gypsum | 5 t/ha, incorporated to depth of 20-40 cm |
3 | Deep liquid NPK | Incorporated to depth of 20-40 cm, N to match chicken manure |
4 | Deep chicken manure | 8 t/ha, incorporated to depth of 20-40 cm |
5 | Deep pea straw | 15 t/ha, incorporated to depth of 20-40 cm |
6 | Deep pea straw +gypsum+NPK | 12 t/ha, 2.5 t/ha, incorporated to depth of 20-40 cm, |
7 | Deep pea straw+NPK | 15 t/ha, incorporated to depth of 20-40 cm |
8 | Deep wheat stubble | 15 t/ha, incorporated to depth of 20-40 cm |
9 | Deep wheat stubble +NPK | 15 t/ha, incorporated to depth of 20-40 cm |
10 | Ripping only | To depth of 40cm |
11 | Surface gypsum | 5 t/ha, applied at soil surface |
12 | Surface chicken manure | 8 t/ha, applied at soil surface |
13 | Surface pea straw | 15 t/ha, applied at soil surface |
At late flowering soil coring was completed using a tractor-mounted hydraulic soil-coring rig and 45 mm diameter soil cores. The break core method was used to estimate rooting depth and exposed roots were recorded at the following depths 0 - 10, 10 - 20, 20 - 40, 40 - 60, and 60 – 100 cm. Quadrat samples of 2m2 were taken at physiological maturity to measure plant biomass and grain yield.
Grogan genotypes screening experiment
In 2019 an experiment was conducted near the township of Grogan in southern NSW, which included 17 commercial wheat genotypes in a row column design with four replicates. The soil profile was slightly acidic in the top 10cm (pH1:5 water 5.9) and pH dramatically increases with depth (Table 1). The changes in soil sodicity (exchangeable sodium percentage, ESP) followed a similar trend of soil pH with ESP at 10.5% in the topsoil and increasing up to 40% in the subsoil (Table 1).
Table 3. Site characterisation for the Grogan experimental site. Values are means (n=5).
Soil depths (cm) | EC | pH (1:5 water) | Colwell-P | CEC (cmol(+)/kg) | Exchangeable sodium percentage |
|---|---|---|---|---|---|
0-10 | 309.40 | 5.87 | 58.80 | 16.66 | 10.53 |
10-20 | 133.00 | 7.65 | 7.40 | 22.06 | 11.97 |
20-30 | 136.90 | 8.76 | 2.62 | 24.53 | 15.94 |
30-40 | 207.66 | 9.12 | 2.50 | 25.55 | 20.12 |
40-60 | 338.94 | 9.60 | 1.34 | 27.17 | 26.27 |
60-80 | 530.40 | 9.53 | 1.00 | 31.63 | 36.68 |
80-100 | 897.20 | 9.43 | 1.48 | 34.07 | 40.25 |
100-120 | 1148.20 | 9.38 | 1.50 | 35.28 | 40.35 |
The experiment was sown on 17 May 2019 using a direct sown drill with DBS tynes spaced at 25cm. At sowing 90 kg MAP (20kg P/ha and 9kg N/ha) was drilled in all plots and 75 kg N/ha was surface applied just prior to stem elongation. Mean plant density as measured by seedling counts at four weeks after sowing was 116 ± 1.6 (mean ± SE of 68 plots) plants m-2. At different growth stages multispectral images (MicaSense RedEdge-MX) were collected using drone technology to determine different vegetation indices such as normalised differences in vegetation index (NDVI) and leaf chlorophyll index (LCI) as a surrogate of canopy attributes and plant physiological processes (Liu et al., 2019; Satir and Berberoglu, 2016; Zhang et al., 2019). Quadrat samples of 1m2 area were taken at physiological maturity to measure plant biomass and grain yield. Harvest index was calculated as grain yield divided by biomass.
Results
Rand amendment trial
The one-off application of various amendments (Table 2) significantly affected the crop grain yield over 4 consecutive years. For example, in 2020, wheat grain yield (relative to control) increased following the deep placement of wheat stubble, wheat stubble + nutrient and gypsum by 21%, 20 and 18% respectively (P < 0.001) (Figure 2). The variations in yield in response to surface application of amendments or ripping only was not significantly different from the control. A multi-year cumulative analysis of grain yield response (2017-2020) indicated that deep placement of plant-based stubble, gypsum and their combination resulted in significant and consistent improvements in crop yield (Table 4). A preliminary cumulative gross return is also presented in Table 4.
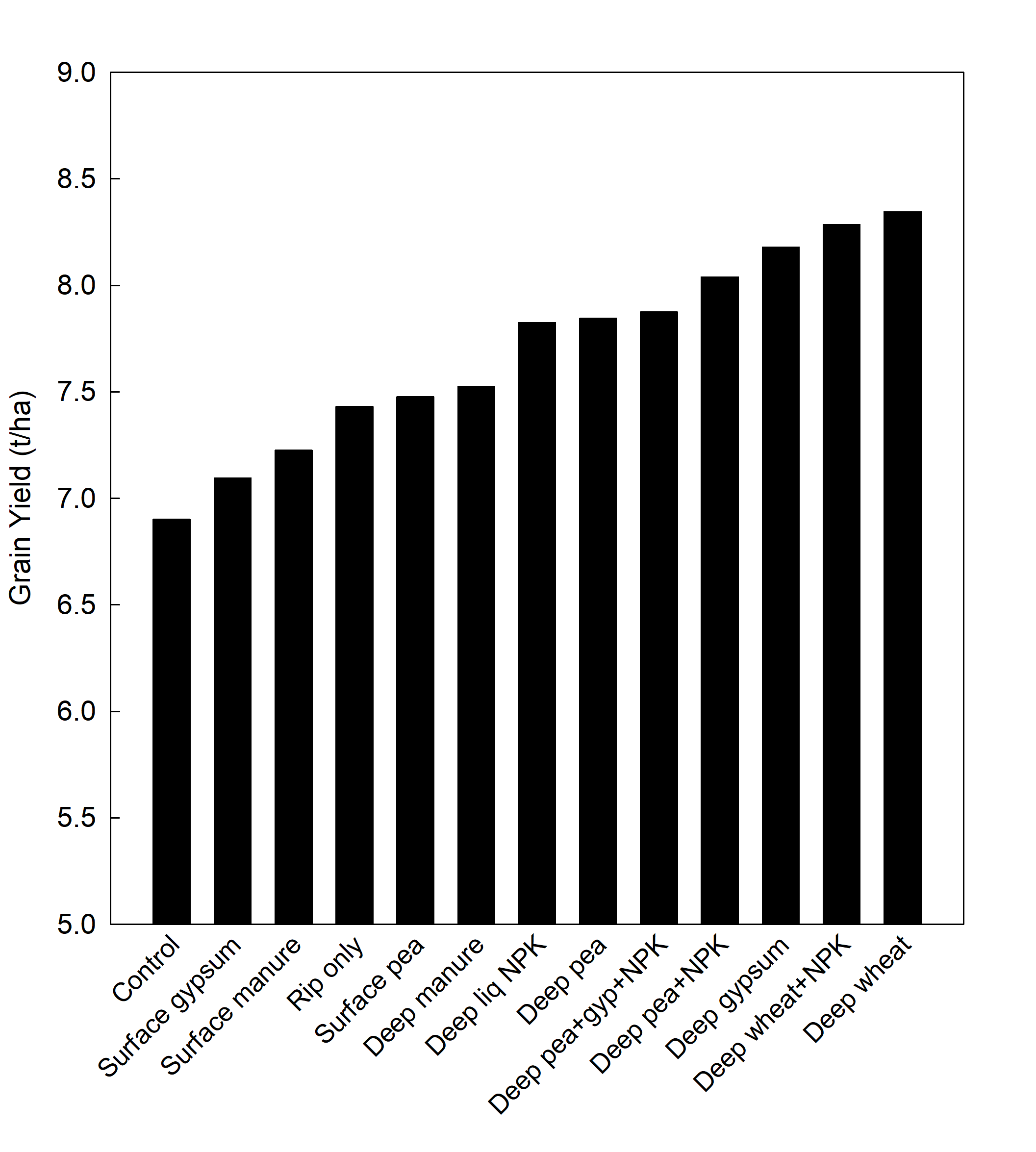 Figure 2. The mean effect of surface or deep-placed amendments on grain yield of wheat (cv. Scepter) grown in an alkaline dispersive subsoil in Rand, SNSW in 2020. Values are mean (n=4). LSD0.05 = 0.67.
Figure 2. The mean effect of surface or deep-placed amendments on grain yield of wheat (cv. Scepter) grown in an alkaline dispersive subsoil in Rand, SNSW in 2020. Values are mean (n=4). LSD0.05 = 0.67.
Table 4. Cumulative grain yield (2017-2020) and cumulative gross return ($) for barley (2017; $220/t), wheat (2018; $250/t), canola (2019; $600/t) and wheat (2020; $250/t) at Rand.
Treat | Yield (t/ha) | $ | ||
|---|---|---|---|---|
Control | 15.3 | a | 4517 | a |
Surface gypsum | 15.5 | a | 4576 | a |
Rip only | 15.9 | ab | 4737 | ab |
Surface pea | 16.00 | ab | 4817 | ab |
Deep liq NPK | 17.1 | bc | 4847 | ab |
Surface manure | 17.1 | bc | 5104 | bc |
Deep wheat | 18.2 | cd | 5388 | cd |
Deep manure | 18.3 | cd | 5428 | cd |
Deep pea+NPK | 18.4 | cd | 5557 | d |
Deep wheat+NPK | 18.5 | d | 5383 | cd |
Deep pea | 18.7 | d | 5507 | d |
Deep gypsum | 18.9 | d | 5684 | d |
Deep pea+gyp+NPK | 19.4 | d | 5699 | d |
Over the course of this study several key measurements of soil and crop parameters were made to investigate the impact of various amendments on soil:plant interactions.
The number of visible roots in the amended subsoil layer (20 – 40cm depth) were significantly (P < 0.05) affected by different amendments (Figure 3). Deep placement of both manure and pea hay increased the number of visible roots by more than 3-fold. Neutron probe readings taken in September also indicate that the highest root counts were associated with the driest soil water profile (Figure 4). Variation in soil pH measured at the amended layer is shown in Table 5. Compared to the control, deep placement of gypsum reduced the soil pH by 0.86 units (8.99 to 8.13) at 20 – 40cm depth. However, pH was not affected by other treatments.
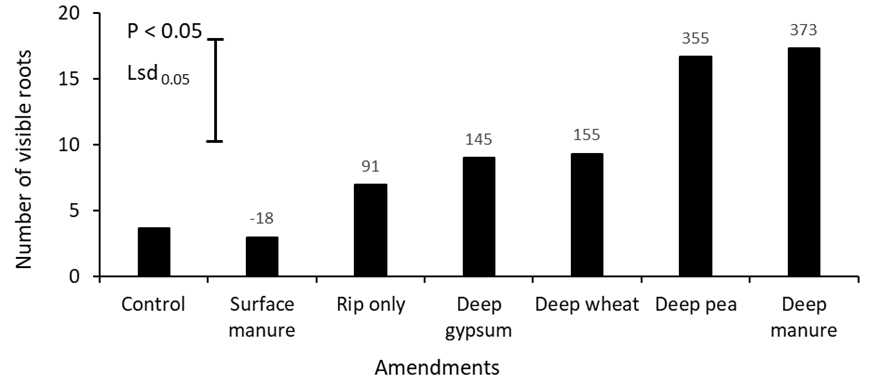 Figure 3. The mean effect of surface or deep-placed amendments on the number of visible roots at 30cm at late flowering of canola (cv. Pioneer® 45Y91CL) grown in alkaline dispersive subsoil in Rand, SNSW in 2019. Values on the top of each bar is representing percent change of visible roots compared to control.
Figure 3. The mean effect of surface or deep-placed amendments on the number of visible roots at 30cm at late flowering of canola (cv. Pioneer® 45Y91CL) grown in alkaline dispersive subsoil in Rand, SNSW in 2019. Values on the top of each bar is representing percent change of visible roots compared to control.
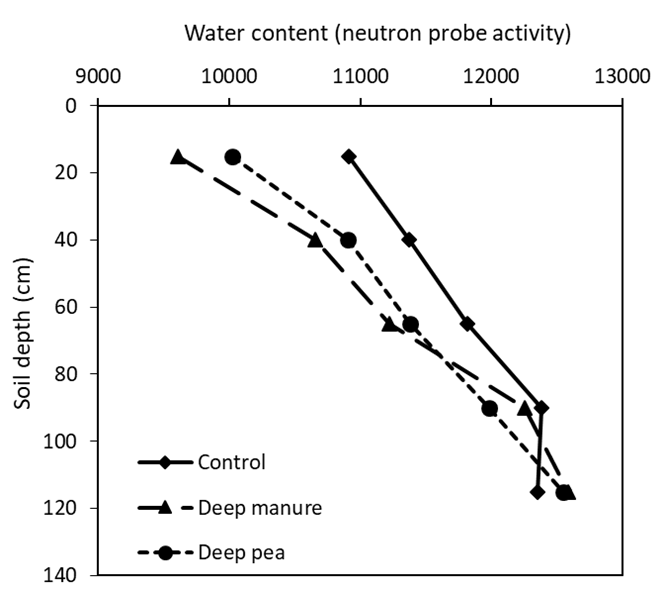
Figure 4. Neutron probe readings taken in September at the Rand amendment site for contrasting treatment comparisons. Results are based on the neutron activity (raw data) where higher values represent higher water content in the soil profile. Values are averages (n = 4).
Table 5. The changes in soil pH (20-40 cm) in selected treatments at the Rand site. Samples were collected in May 2020. LSD0.05 = 0.27.
Amendment | Predicted mean | Group |
|---|---|---|
Control | 8.99 | a |
Deep liq NPK | 8.96 | a |
Rip only | 8.94 | a |
Deep wheat+NPK | 8.93 | ab |
Surface gypsum | 8.92 | ab |
Deep pea | 8.87 | ab |
Deep wheat | 8.83 | ab |
Deep manure | 8.60 | bc |
Deep pea+gyp+NPK | 8.52 | c |
Deep gypsum | 8.13 | d |
Grogan genotypes screening trial
Significant (P < 0.001) genotypic variation occurred in grain yield among the genotypes and ranged from only 0.57 t/ha (Gregory) to 2.0 t/ha (Scepter, Emu Rock and Mace; Figure 5). Biomass at final harvest did not significantly differ among the genotypes (data not shown; P = 0.11) and there was no significant (P = 0.09) correlation between grain yield and biomass at final harvest (Figure 6).
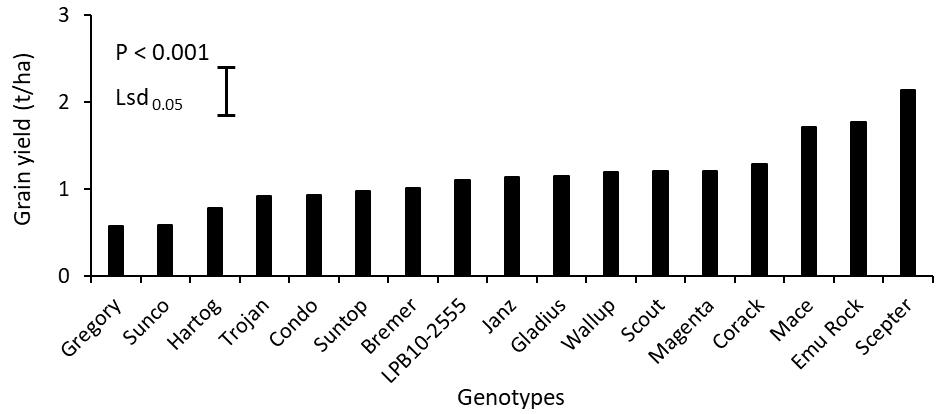 Figure 5. Variations in grain yield of 17 wheat genotypes grown in alkaline sodic dispersive subsoil in Grogan, SNSW in 2019. Each data point is mean values of n = 4.
Figure 5. Variations in grain yield of 17 wheat genotypes grown in alkaline sodic dispersive subsoil in Grogan, SNSW in 2019. Each data point is mean values of n = 4.
(Varieties Gregory, Trojan, Condo, Suntop, Bremer, Gladius, Wallup, Scout, Magenta, Corack, Mace, Emu Rock and Scepter are protected under the Plant Breeders Rights Act 1994)
Significant variation was observed in harvest index (data not shown; P < 0.001), which ranged from 0.08 (Gregory) to 0.26 (Scepter). A significant (P < 0.001) and positive correlation between harvest index and grain yield is observed among the studied genotypes (Figure 6).
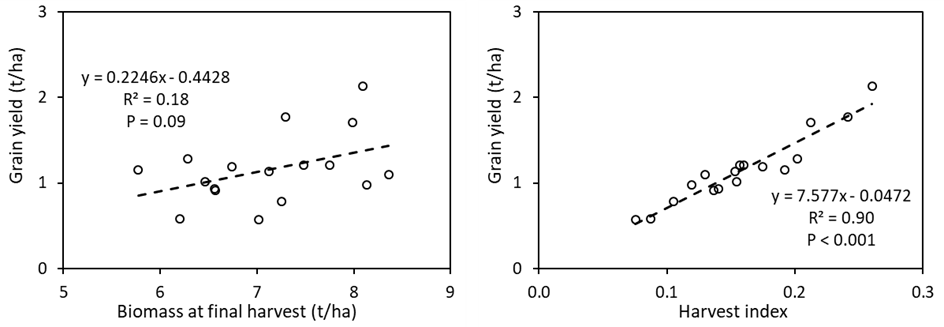
Figure 6. Linear regressions between grain yield and biomass at final harvest (left) and harvest index (right) of 17 wheat genotypes grown in alkaline sodic dispersive subsoil at Grogan, SNSW in 2019.
All the non-destructive vegetation indices, i.e. NDVI (P < 0.01), NDRE (P < 0.001) and LCI (P < 0.001) measured at stem elongation showed significant and positive correlation with biomass at anthesis (Figure 7).
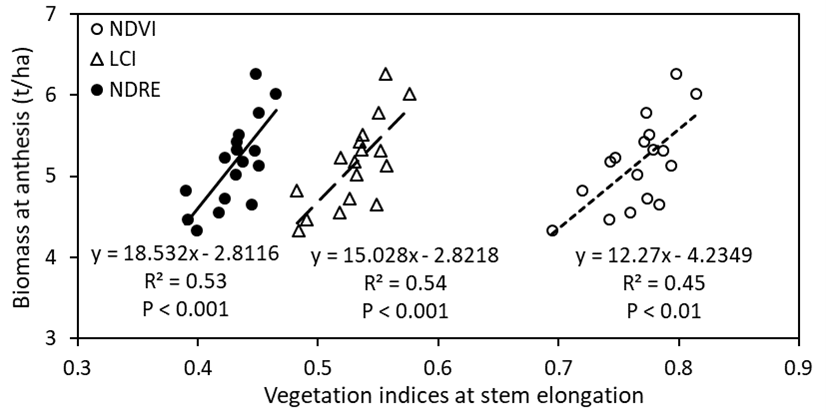 Figure 7. Linear regressions between vegetation indices (measured at stem-elongation) and anthesis biomass of 17 wheat genotypes grown in alkaline sodic dispersive subsoil at Grogan, SNSW in 2019. NDVI = normalised differences in vegetation index; LCI = Leaf chlorophyll index; NDRE = Normalised difference red edge.
Figure 7. Linear regressions between vegetation indices (measured at stem-elongation) and anthesis biomass of 17 wheat genotypes grown in alkaline sodic dispersive subsoil at Grogan, SNSW in 2019. NDVI = normalised differences in vegetation index; LCI = Leaf chlorophyll index; NDRE = Normalised difference red edge.
Discussion
In Alkaline dispersive soils, several properties of subsoils including, high pH, high levels of soluble carbonate species, poorly structured dense clay, and dispersion together with overall poor chemical fertility, represent a hostile environment for crop roots. Here we demonstrate the impact of various amendments on these properties and the potential to re-engineer these hostile subsoils for improved crop performance.
Barley, wheat, canola and wheat were grown in 2017–2020, respectively, under increasingly dry conditions. Growing season rainfall (April to November total) was average in 2017 (decile 5), and declined in 2018 (decile 1.5), with still drier conditions in 2019 (decile 1.0), when only 45 mm of rain (decile 0) fell during the spring months from September to November. This improved in 2020 where the trial received 401 mm during growing the season. The amendments that consistently resulted in significant yield increases above the control, were the deep-placed combination of pea straw pellets, gypsum and liquid fertilizer nutrients, and the deep-placed gypsum and deep placed pea straw (Table 4). Improvements in subsoil structure were measured in the winter of 2019. The deep crop residue amendments significantly increased macro aggregation, as measured on the rip-line at a depth of 20-40 cm. Similarly, deep gypsum and the deep gypsum/pea straw/nutrient combination markedly increased water infiltration into the soil profile, with higher saturated hydraulic conductivities measured on the rip-line. Our results to date indicate that independent modes of action of various amendments (e.g. crop residue vs gypsum) are required in the amendment mix, in order to ameliorate these subsoils. For example, adding gypsum reduced pH in the amended subsoil to below 8.5 (Table 5). This indicates that significant changes in soil pH can occur with realistic application rates of gypsum in subsoil. Given high alkalinity also increases negative charges on the surfaces of clay particles (Rengasamy et al., 2016), which increases clay dispersion, a reduction in pH following gypsum application also resulted in significant improvement (reduction) in soil dispersion (Tavakkoli et al., 2015). In alkaline sodic soils, high ESP and high pH are always linked together and it is difficult to apportion their effects on the resulting poor soil physicochemical conditions and consequently on crop growth.
The addition of pea straw and nutrients provides substrate for enhanced biological activity resulting in increased macro aggregation and improved subsoil structure. When combined together, organic and inorganic amendments may result in additive effects to improve soil physical and chemical properties (Fang et al., 2020a; Fang et al., 2020b).
In a year of intensive drought like 2019, the grain yield improvements at Rand may be attributed to the additional root growth in the amended subsoil layer (Figure 3), which facilitated the use of extra subsoil water (Tavakkoli et al., 2019 and Figure 4). Under dryland conditions, water captured by roots in the subsoil layer is extremely valuable as its availability coincides with the grain filling period and has a very high conversion efficiency into grain yield (Kirkegaard et al., 2007; Wasson et al., 2012). A major focus of this current research is to understand the amelioration processes of the subsoil application of organic and inorganic amendments. A tentative, but promising, finding from our field and controlled environment trials, is that farm grown products like wheat and pea stubbles when mixed with nutrients improve soil aggregation, root growth, water extraction and grain yield and these treatments are comparable to animal manures and gypsum. If confirmed, this means that grain growers have a potentially large supply of relatively inexpensive organic ameliorants already available in their paddocks, which will increase the application options and viability of correcting subsoil sodicity.
Despite, demonstrating significant improvement in grain yield with subsoil incorporation of organic and inorganic amendments, the widespread adoption of these practices are still limited by their cost effectiveness. Identifying traits associated with the superior tolerance to different soil constraints may be a low cost technique to tackle this issue (McDonald et al., 2012). Given the intensive drought condition in the study year, considerable genotypic variation was observed with some varieties having 3- fold higher grain yield than other varieties. Based on controlled-environment studies, the high yielding varieties at Grogan, ‘Mace and Emu Rock,’ are moderately tolerant to tolerant to high pH and have roots that can grow relatively well through soils of high bulk density, whereas low yielding varieties such as Gregory, Hartog and Sunco, are more sensitive to one or both of these stresses. The very low harvest index in the trial suggests that there was severe stress around flowering to reduce grain set, as well as during grain filling. Results suggest that the ability to maintain root growth hay have helped to alleviate stress in varieties like Emu Rock and Mace. Furthermore, different traits associated with this greater yield performance of wheat genotypes are crucial aspects of future breeding programs.
Conclusions
The findings from the current field studies demonstrate initial but promising results of ameliorating alkaline dispersive subsoils in medium and high rainfall zones of southern NSW. Deep placement of organic and inorganic amendments resulted in significant yield improvement in four successive years at Rand where subsoil water was present. This yield improvement was facilitated by a reduction in soil pH and ESP% and increased microbial activity that can lead to improved soil aggregation. Furthermore, deep placement of organic and inorganic amendments increased root growth, which in turn increased soil water use from the deeper clay layers during the critical reproductive stages of crop development, thereby increasing grain yield. In addition to soil management, genotypic variability in grain yield of wheat cultivars observed and their associated traits identified in the current study can be used for improving wheat germplasm through future breeding programs.
References
Adcock, D., McNeill, A. M., McDonald, G. K., and Armstrong, R. D. (2007). Subsoil constraints to crop production on neutral and alkaline soils in south-eastern Australia: a review of current knowledge and management strategies. Australian Journal of Experimental Agriculture 47, 1245-1261.
Fang, Y., Singh, B. P., Collins, D., Armstrong, R., Van Zwieten, L., and Tavakkoli, E. (2020a). Nutrient stoichiometry and labile carbon content of organic amendments control microbial biomass and carbon-use efficiency in a poorly structured sodic-subsoil. Biology and Fertility of Soils 56, 219-233.
Fang, Y., Singh, B. P., Farrell, M., Van Zwieten, L., Armstrong, R., Chen, C., Bahadori, M., and Tavakkoli, E. (2020b). Balanced nutrient stoichiometry of organic amendments enhances carbon priming in a poorly structured sodic subsoil. Soil Biology and Biochemistry 145, 107800.
Farooq, M., Hussain, M., and Siddique, K. H. M. (2014). Drought stress in wheat during flowering and grain-filling periods. Critical Reviews in Plant Sciences 33, 331-349.
Gill, J. S., Sale, P. W. G., and Tang, C. (2008). Amelioration of dense sodic subsoil using organic amendments increases wheat yield more than using gypsum in a high rainfall zone of southern Australia. Field Crops Research 107, 265-275.
Hajkowicz, S., and Young, M. (2005). Costing yield loss from acidity, sodicity and dryland salinity to Australian agriculture. Land Degradation & Development 16, 417-433.
Isbell, R. F. (2002). "The Australian Soil Classification.," CSIRO, Melbourne.
Kirkegaard, J. A., Lilley, J. M., Howe, G. N., and Graham, J. M. (2007). Impact of subsoil water use on wheat yield. Australian Journal of Agricultural Research 58, 303-315.
Liu, C., Liu, Y., Lu, Y., Liao, Y., Nie, J., Yuan, X., and Chen, F. (2019). Use of a leaf chlorophyll content index to improve the prediction of above-ground biomass and productivity. PeerJ 6, e6240-e6240.
McDonald, G. K., Taylor, J. D., Verbyla, A., and Kuchel, H. (2012). Assessing the importance of subsoil constraints to yield of wheat and its implications for yield improvement. Crop & Pasture Science 63, 1043-1065.
McDonald, G. K., Taylor, J. D., Verbyla, A., and Kuchel, H. (2013). Assessing the importance of subsoil constraints to yield of wheat and its implications for yield improvement. Crop and Pasture Science 63, 1043-1065.
Northcote, K. H., and Skene, J. K. M. (1972). "Australian SoiIs with Saline and Sodic Properties," CSIRO, Melbourne, Australia.
Nuttall, J. G., Hobson, K. B., Materne, M., Moody, D. B., Munns, R., and Armstrong, R. D. (2010). Use of genetic tolerance in grain crops to overcome subsoil constraints in alkaline cropping soils. Australian Journal of Soil Research 48, 188-199.
Orton, T. G., Mallawaarachchi, T., Pringle, M. J., Menzies, N. W., Dalal, R. C., Kopittke, P. M., Searle, R., Hochman, Z., and Dang, Y. P. (2018). Quantifying the economic impact of soil constraints on Australian agriculture: A case-study of wheat. Land Degradation & Development 29, 3866-3875.
Passioura, J. B., and Angus, J. F. (2010). Chapter 2 - Improving Productivity of Crops in Water-Limited Environments. In "Advances in Agronomy" (D. L. Sparks, ed.), Vol. 106, pp. 37-75. Academic Press.
Rengasamy, P. (2002). Transient salinity and subsoil constraints to dryland farming in Australian sodic soils: an overview. Australian Journal of Experimental Agriculture 42, 351-361.
Rengasamy, P., Tavakkoli, E., and McDonald, G. K. (2016). Exchangeable cations and clay dispersion: net dispersive charge, a new concept for dispersive soil. European Journal of Soil Science 67, 659-665.
Sale, P., Tavakkoli, E., Armstrong, R., Wilhelm, N., Tang, C., Desbiolles, J., Malcolm, B., O'Leary, G., Dean, G., Davenport, D., Henty, S., and Hart, M. (2021). Chapter Six - Ameliorating dense clay subsoils to increase the yield of rain-fed crops. In "Advances in Agronomy" (D. L. Sparks, ed.), Vol. 165, pp. 249-300. Academic Press.
Sale, P. W., Gill, J. S., Peries, R. R., and Tang, C. (2019). Crop responses to subsoil manuring. I. Results in south-western Victoria from 2009 to 2012. Crop and Pasture Science 70, 44-54.
Satir, O., and Berberoglu, S. (2016). Crop yield prediction under soil salinity using satellite derived vegetation indices. Field Crops Research 192, 134-143.
Tavakkoli, E., Rengasamy, P., Smith, E., and McDonald, G. K. (2015). The effect of cation–anion interactions on soil pH and solubility of organic carbon. European Journal of Soil Science 66, 1054-1062.
Tavakkoli, E., Weng, Z. H., Tahmasbian, I., Uddin, S., Poile, G., Oates, A., Xu, B. B., Sandral, G., Fang, Y., and Armstrong, R. (2019). Understanding the amelioration processes of the subsoil application of amendments. 18 - 19 February 2019, GRDC Grains Research Update (Wagga Wagga).
Wasson, A. P., Richards, R. A., Chatrath, R., Misra, S. C., Prasad, S. V. S., Rebetzke, G. J., Kirkegaard, J. A., Christopher, J., and Watt, M. (2012). Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. Journal of Experimental Botany 63, 3485-3498.
Zhang, K., Ge, X., Shen, P., Li, W., Liu, X., Cao, Q., Zhu, Y., Cao, W., and Tian, Y. (2019). Predicting rice grain yield based on dynamic changes in vegetation indexes during early to mid-growth stages. Remote Sensing 11, 387.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support. This research was undertaken as part of projects DAV00149 and UA000159.
Contact details
Dr Ehsan Tavakkoli
NSW DPI, Wagga Wagga Agricultural Institute
Phone: 02 69381992
Email: Ehsan.tavakkoli@dpi.nsw.gov.au
Twitter handle: @EhsanTavakkoli
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
® Registered trademark
Was this page helpful?
YOUR FEEDBACK
