Bacterial ice nucleation activity in rainfall and on crop residues may explain why pre-frost rainfall and stubble retention increase frost damage in WA cropping systems
Bacterial ice nucleation activity in rainfall and on crop residues may explain why pre-frost rainfall and stubble retention increase frost damage in WA cropping systems
Author: Ben Biddulph (DPIRD South Perth), Amanuel Bekuma (DPIRD South Perth), Sarah Jackson (DPIRD South Perth), Chaiyya Cooper (Curtin University), Rebecca Swift (Curtin University) and Dean Diepeveen (DPIRD South Perth) | Date: 14 Apr 2021
Key messages
- In-field thermography after head emergence indicates canopies freeze from the ground up. Ice nucleation starts first on the stubble and crop residue in the interrow, before moving to the older senesced leaves and up the plant leaf sheath, stems and reproductive tissue.
- Increasing concentrations of ice nucleating protein applied before frost events increased frost damage in a dose response manner, but a wet canopy by itself did not increase frost damage.
- Spring rainfall has biological ice nucleation activity but this varied with site and frost event with the highest activity in the central and upper Great Southern in rainfall events <10mm.
- Rainfall before frost events and retained stubble appeared to increase frost damage due to greater activity of biological ice nucleation. This caused freezing damage to occur earlier in the night resulting in more terminal damage.
Aims
To better understand the mechanism by which stubble retention and rainfall before frost events increase frost damage.
Introduction
Agronomic, genetic and climatic research indicates a very weak correlation between air temperature and frost damage. Trials and treatments that experience similar temperatures often have very different levels of frost damage and these inconsistent results regularly occur within a season at different sites and from year to year at the same site. One possibility that has not been explored within the WA cropping system is whether ice nucleation bacteria (INB), either bought in by in rainfall with the weak frontal systems before frost events or in situ in crop residue and dispersed by rainfall and/or actively growing on the plant canopy, could be responsible for the increased sensitivity of cereal plants to frost.
Two bacterial genera, Pseudomonas syringae and Pantoea agglomerans (formerly Erwinia herbicola), found to be positive for ice nucleating activity (Kozloff et al 1983; Newton & Heyward 1986; Mazarei & Kerr 1987) are present in the WA cropping landscape. The implication within a cropping environment is that, in the presence of these bacteria, water and plant tissue can freeze at temperatures well above what they would normally freeze at, causing cell damage, desiccation and death and resulting in significant yield loss. Without these ice nucleating agents, a plant may remain supercooled with no damage to the reproductive and vegetative parts. Even frost-sensitive plants grown under a glasshouse environment can supercool to between -8°C and -10°C with mild injury (Lindow 1983).
Past frost and stubble management trials have found differences in frost severity, duration and damage (Jenkinson et al 2014; Smith et al 2017 and Biddulph et al 2019). However, the cause-effect relationships have not been established. For instance, stubble retention even with low stubble loads (1t/ha) can increase frost damage in wheat (Biddulph et al 2019). However, it is not known whether stubble increases the frost damage, which in turn increases frost severity and duration, or whether the stubble increases the frost severity and duration, thereby increasing the damage. The mechanism by which stubble increases frost risk in wheat crops remains to be investigated and is the subject of this work.
Method
In-field thermography
To determine where and when frost damage occurs as a result of ice nucleation and where and when freezing starts in wheat canopies, in-field thermography was done on nights with forecast frost events in the spring of 2019 and 2020. A thermal camera (FLIR-T62101 in 2019 and FLIRAX5 in 2020) was set up to observe the stubble/crop residue in the interow as well as the stems and spikes of ~30 tillers. Plants were targeted for the most frost susceptible stages from early head emergence to early grain set (~Z49-71). In 2019, a thermal image was captured every second, and in 2020 an image was captured every minute from ~ midnight to sunrise. Manual interpolation of the thermal images was used to determine the source of ice nucleation and movement of the freezing process. A machine learning approach to optimise images and identify freezing is ongoing.
Rainwater ice nucleation activity
Rainwater was collected in August, September and early October by 20 collaborating growers throughout frost-prone areas of the Great Southern and South Coast in 2018, 2019 and 2019. Rainwater up to a maximum of 50mL was collected from normal rainwater gauges at the time of daily rainfall manual recordings (~07:00-09:00am), samples were immediately frozen and stored at -20°C until processing. The Ice Nucleating Activity (INA) of the rainwater samples was determined in Dec 2020 by droplet freezing assay as described by Vali and Stansbury (1965) but with minor modifications. A total of 20 droplets (10µl each) of rainfall were aliquoted on a parafilm floating on a temperature-controlled cooling bath pre-chilled at 0°C. The temperature of the cooling bath was lowered by 1°C every five minutes and thermographic camera (FLIR-T62101) was used to capture the freezing events of the droplets. The average temperature required to freeze 50% of the droplets (Ice Nucleating Temperature INT50) was then determined.
Dose response to ice nucleating proteins
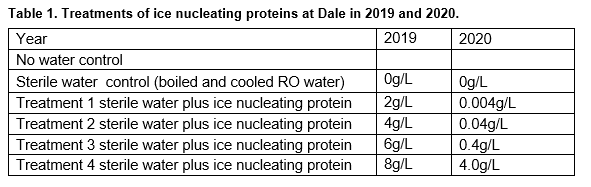
Trials were sown into a flat, frost-susceptible part of a paddock with a history of multiple frost events. Sceptre wheat was sown using local agronomic practices, including crop rotation, but prophylactic fungicides were applied at stem elongation and flag leaf emergence to minimise foliar diseases. The trials consisted of yield plots with six sowing dates ranging from ~ 15 April to 25 May to expose plants to reproductive frost damage throughout the frost window of August to September in 2019 and 2020. Before a forecast frost event (minimum temperature forecast of 2°C) or lower, the sowing dates between ear peep and early grain set (Z49-71) were sprayed with various rates of ice nucleating protein (Table 1) until the canopy was at dripping point. This rate equates to ~0.5 mm of equivalent rainfall. There were two controls – a dry canopy control of no water and a wet canopy control of sterile water (0.0g/L). The canopy was sprayed late in the afternoon between 4–6pm to minimise the amount of UV light capcable of degrading the ice nucleating protein (time point sampling indicates the protein breaks down by midday the following day if no cloud cover and within 2-3 days if overcast/partial cloud cover). There was considerable variability in the response to ice nucleating proteins in 2019.The protein is contained in freeze dried pellets and is not readily water soluble and hence is difficult to keep in suspension. Plots are sprayed in treatment order, (lowest to highest rate) so it is likely the first replicate of plots sprayed may have had a slightly different rate to later replicates, as the suspension settled while being applied in 2019. In 2020, application methods were improved by firstly milling the dry pellets before mixing, using larger water volumes in the initial serial dilutions and continuous agitation while applied. Tiny Tag temperature loggers (TGP-4017) were installed within each plot to measure canopy temperature at 600mm every 15 minutes from canopy closure through to senescence. Changes in frost severity and duration were made when a single logger at a site recorded a sub-zero temperature from the susceptible window defined as Z45-85. To assess crop emergence, plant counts were done three weeks after sowing. From Zadok (Z) 40 (flag-leaf sheath extending) onwards, plots were assessed weekly for crop developmental stage. At Z85 (late dough) 30 heads were collected from three locations near temperature sensors for floret sterility (FS) assessments, irrespective of whether frosts had occurred or not. Biomass cuts were collected at Z89 (hard dough) for harvest index, 1000-grain weight, hectolitre weight and screenings. At maturity, final grain yield was determined with a plot-header.
Statistical analysis
Floret sterility, harvest index and grain yield were analysed by ANOVA and LSD0.05 used to compare the means of the four replicates. Values presented are the predicted means plus and minus standard errors. ANOVA indicated where treatment effects were not significant (NS) at P<0.05.
Results and Discussion
Infield thermography
In-field thermography during frost events in 2019 and 2020 after head emergence indicates the canopies froze from the ground up. A typical example is displayed in Image 1. Ice nucleation seen as a rise in temperature associated with the latent heat of freezing (seen as white plant parts indicated with arrows) started first on the stubble and crop residue in the interow, before moving to the older senesced leaves and up the plant leaf sheath, stems and spikes (Image 1). The freezing process was also observed to be a gradual process with it taking ~20–30min for a plant to freeze from the ground up to the spike. A machine learning based approach to optimise and merge digital and thermal images and identify heat exotherms/freezing is ongoing. Our lab results on ice nucleation activity of various tissues confirm the likely order of freezing (Bekuma et al 2021) and indicate that older leaves are the primary sites of ice nucleation (-4.7°C) followed by stubble (-5.7°C). This increases the risk of frost damage during the most susceptible stages. The higher INA on the stubble and older leaves is likely to be caused by ice-nucleating bacteria (INB), known to cause frost damage at temperatures as warm as -5°C.
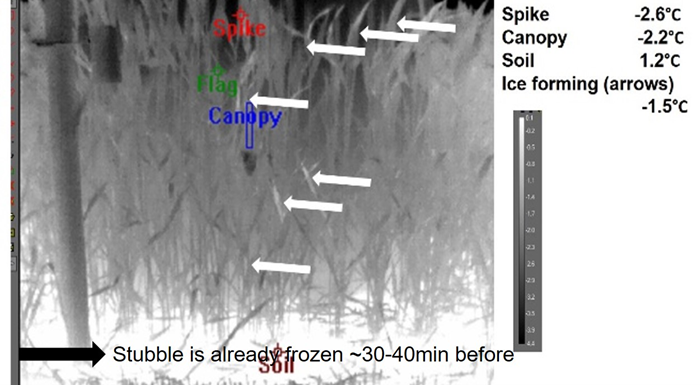
Image 1. Infra-red thermography image from the infected stubble treatment at Dale Research Station on 6 September 2019 at around 5:30 am. Arrows indicate a heat exotherm or increase (rise in temp) associated with the ice nucleation and freezing of the plant tissue. No freezing was observed in the ‘no-stubble’ treatment (images not shown).
Dose response to ice nucleating proteins
To determine if ice nucleating proteins either from crop residue, see Bekuma et al(2021) for more details, or present in rainfall can increase frost damage, ice nucleating proteins mixed at various rates in sterile water were applied to plants in the late afternoon on nights with forecast frost events when each time of sowing was between ear peep (Z49) and early grain set (Z71). In 2019 this resulted in each sowing date from 17 April onwards receiving several applications and there was a clear increase in visual symptoms of stem, head, flower and early grain set frost damage in the treated plots. The stem frost damage symptoms were consistent with those seen when crops have rain on them in the late afternoon/early evening before a frost event and these symptoms were not observed in the untreated controls. When there was 10-60% floret sterility in the ‘no water’ control, the ice nucleating proteins increased frost damage (17 April to 17 May sowing dates). There was no frost damage in the ‘no water’ control on 10 April in 2019, while on 24 April, the frost damage in the controls was >60% and although the ice nucleating protein increased this, it was not statistically different to the controls. The increase in floret sterility with the ice nucleating proteins was also associated with a lower harvest index and lower grain yields (Figure 1) while biomass and 1000-grain weight remained unaffected (data not shown). Application of sterile water alone (0.0g/L) did not show a significant increase in frost damage over the ‘no water’ control. This suggests an ice nucleator must be present to increase frost damage, not just wetting the canopy.
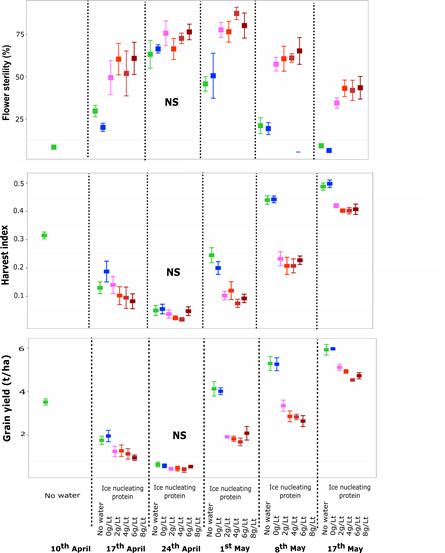
Figure 1. Floret sterility (a), harvest index (b) and grain yield (c) of Sceptre wheat at Dale 2019 as affected by application of ice nucleating protein at four different rates (2,4,6,8 g/L) vs the two controls (no water and sterile water; 0.0g/L) across different times of sowing. Values are the mean of 4 reps +/- SE.
In 2020, the Dale site experienced few frosts and not all TOS had the ice nucleating protein applied. Again the first sowing date (15 April) was not exposed to frosts and not sprayed and hence data is not presented. On 29 April and 5 May there were several minor frost events which caused ~10% sterility in the controls and the ice nucleating protein was applied before these events. Under these minor frost conditions, application of the ice nucleating protein increased frost damage in a logarithmic manner from ~10% in the controls up to 45% floret sterility with 4g/L on 29 April and 35% on 5 May. The increase in floret sterility was again associated with a reduction in harvest index and grain yield (Figure 2). Again, the application of sterile water alone (0.0g/L) on 29 April and 5 May did not cause a significant increase in frost damage over the control suggesting an ice nucleator must be present in the sterile water to increase frost damage, not just wetting the canopy. As there was no frost forecast (<2°C) when the canopy was in the susceptible window (Z45-71) the ice nucleating protein was not applied to the next sowing date (floret sterility, HI and grain yield not shown) so the forecast temperature threshold was relaxed to an overnight minimum of <4°C for the last two sowing dates (19 and 26 May) in case there was a frost. This resulted in several applications, but none of these nights actually eventuated in a frost and these times of sowing were not frosted (floret sterility <10% in all treatments). Without frost there was no effect of the ice nucleating proteins on sterility, harvest index or grain yield indicating that applying the proteins or sterile water on their own do not cause any effects on the crop.
In summary, with no frost there is no effect of ice nucleating proteins, with light frost (~10-15% floret sterility) and with moderate frost (~20-80% floret sterility), ice nucleating proteins increase frost damage to moderate and severe levels. Without frost or with severe frost (>60% floret sterility), the ice nucleating proteins did not make the frost worse, possibly because there was already sufficient “natural” ice nucleating proteins from native P.syringae.
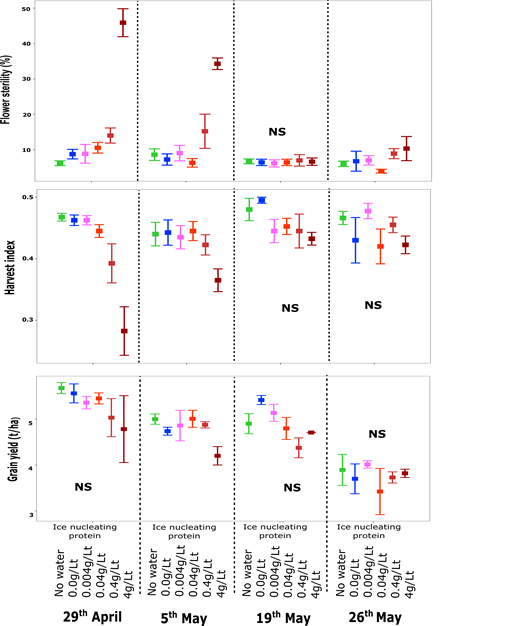
Figure 2. Floret sterility (a), harvest index (b) and grain yield (c) of Sceptre wheat at Dale 2020 as affected by application of ice nucleating protein at four different rates (0.004, 0.04, 0.4 and 4 gm/Lt) and controls (no water, and sterile water;0.0 g/L) across different time of sowing. Values are the mean of 4 reps +/- SE.
Ice nucleation activity of spring rainfall
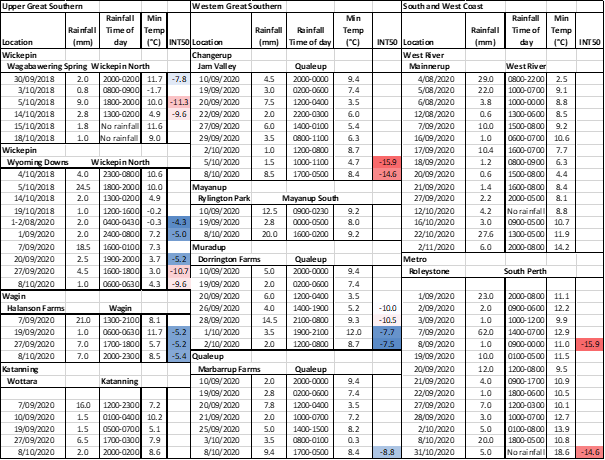
Spring rainfall collected across the frost-prone areas of the Great Southern and South Coast wheat belt were assayed for ice nucleation activity. Of the 175 samples assayed, there was ice nucleating activity in 20 of the samples. Of the 20 samples with activity, 16 of these were from rainfall events of <5mm (16 out of 97 samples <5mm). Of these 16, the samples with the highest activity (INT50 >-8°C) were in rainfall events which fell in the late afternoon or early evening in late September (26-27th Sept) and early October (8th Oct) in 2020 at all 4 sample locations in the western and upper Great Southern (4 sites within 150km of each other at West Kojonup, Wagin and Wickepin Table 3). There was some ice nucleation activity in rainfall events of 5 to 10mm (5 out of 30 samples) (INT50 >-12°C) but less for larger rainfall events >10mm (2 out of 30 samples). There was limited activity in the 2018 and 2019 samples (data not shown), most activity was in the 2020 samples (Table 2). The longer storage time (24-12 months) compared to the 2020 samples may have contributed to this as the storability of rainwater and ice nucleation activity is unknown.
Table 2. Ice nucleation activity of spring rainfall collected through the frost prone areas of the WA wheatbelt in Sept-October 2020 and Wickepin in 2018. Blue shading indicates biological ice nucleating activity red indicates mineral or biological ice nucleating activity.
Conclusion
Rainfall before frost events in combination with retained stubble appears to increase frost damage due to greater ice nucleation activity. This causes freezing damage to occur earlier in the night resulting in more terminal damage. In-field thermography after head emergence indicates canopies freeze from the ground up. Ice nucleation starts first on the stubble and crop residue in the interrow, before moving to the older senesced leaves and up the plant leaf sheath, stems and reproductive tissue. Increasing concentrations of ice nucleating protein applied before frost events increased frost damage by several fold in a dose response manner. However, a wet canopy alone did not increase frost damage. Spring rainfall has biological ice nucleation activity, which varies with site and event, but is likely to be responsible for increased frost damage in commercial crops. Growers should continue to manage frost in the same way with frost zoning, considering enterprise based on frost risk and matching crop type, variety maturity and sowing date to minimise frost exposure. Further research needs to identify other management practices and techniques (other than stubble removal) that could control ice nucleating bacteria and their associated increase in frost damage.
Acknowledgments
This work was supported by the Council of Grain Grower Organisations and contributing growers, DPIRD, WA the trial site hosts Bill, Anne Cleland and families, Dale WA and many growers and consultants throughout WA who provided rainwater samples.
Paper reviewed by: George Mwenda, DPIRD and Steve Curtin, ConsultAg
Contact details
Ben Biddulph
DPIRD 3 Baron-Hay Court South Perth
Ph: 0428 920 654
Email: ben.biddulph@dpird.wa.gov.au
References
Bekuma, A., Biddulph, B., Jackson, S., Ryan, K. and Diepeveen, D. (2021). Stubble and senesced leaves are the main sources of ice nucleation activity in wheat, in 2021 GRDC Crop Updates, Perth, Western Australia.
Biddulph, B., Smith R., Turner C., Reeves K. and Jackson S., (2019) Stubble load increases frost severity, duration and damage in frost-prone landscapes in south-western Australia. In Proceedings of the 2019 Agronomy Australia Conference, 25 – 29 August 2019, Wagga Wagga, Australia © 2019.
Hollaway G. J. Bretag T. W. (1997) Survival of Pseudomonas syringae pv. pisi in soil and on pea trash and their importance as a source of inoculum for a following field pea crop. Australian Journal of Experimental Agriculture 37, 369-375. https://doi.org/10.1071/EA96095
Jenkinson R and Biddulph, B., (2014) Role of stubble management on the severity and duration of frost and its impact on grain yield, in 2014 GRDC Crop Updates, Perth, Western Australia.
Lindow, S. E., Arny, D. C., & Upper, C. D. (1978). Distribution of ice nucleation-active bacteria on plants in nature. Applied and environmental microbiology, 36(6), 831–838. https://doi.org/10.1128/AEM.36.6.831-838.1978
Lindow S. E., Arny D. C., Upper C. D., 1982. Bacterial Ice Nucleation: A Factor in Frost Injury to Plants. Plant Physiology, 70 (4) 1084-1089
Livingston, D.P., Tuong, T.D., Murphy, J.P. (2017). High-definition infrared thermography of ice nucleation and propagation in wheat under natural frost conditions and controlled freezing. Planta 247, 791–806 (2018). https://doi.org/10.1007/s00425-017-2823-4
Moore, L. W. (1988). Pseudomonas syringae: disease and ice nucleation activity. Ornamentals Northwest, 12(2), 3.
Smith R., Minkey, P., Butcher, T., Hyde, S., Jackson, S., Reeves, K., Biddulph, B., (2017). Stubble management recommendations and limitations for frost prone landscapes, in 2017 GRDC Crop Updates, Perth, Western Australia.
Vali, Gabor & Stansbury, E. (1965). Time-dependent characteristics of the heterogenous nucleation of ice. Canadian Journal of Physics. 44. 477-502. 10.1139/p66-044.
