Fungicide resistance in cereals – problem pathogens and strategies to deal with them
Take home messages
- In seasons that favour higher yield potential, one of the most important components in growing high yielding cereal crops is disease management
- Where genetic resistance in a wheat cultivar is not sufficient to delay fungicide decisions until flag leaf emergence (GS37-39), look to target the following three key timings for fungicide intervention; first node GS31, flag leaf emergence GS39 with an optional third application at head emergence GS59
- In barley two timings are essential in order to maximise returns with an option for seed treatment in high disease pressure scenarios. These timings were identified as GS31 and awn tipping (GS49)
- Avoid repeated use of the same fungicide active ingredients, and in the case of the newer Group 11 QoI (strobilurins) and Group 7 SDHIs, where possible restrict strategies to just one application per season in order to slow down and help prevent the selection of resistant strains.
Hyper Yielding Crops research – the importance of disease management
Led by Field Applied Research (FAR) Australia, the Hyper Yielding Crops (HYC) project is a Grains Research and Development Corporation (GRDC) national investment (commenced 1 April 2020) which aims to push the economically attainable yield boundaries of wheat, barley and canola in those regions with higher yield potential. HYC builds on the success of the GRDC’s four-year Hyper Yielding Cereals project in Tasmania, which demonstrated that it is possible to significantly increase yields through sowing the right cultivars and effective implementation of appropriately tailored management strategies. Whilst the project team is clearly aware that Tasmania is not mainland Australia, they believe there are some common threads to the research that could benefit this mainland HYC initiative.
The first is the ability to operate a research centre that can look at all the latest developments in germplasm and agronomy in one location. Five HYC research sites across the higher yielding regions of southern Australia (NSW, WA, SA, VIC and TAS) have been established to engage with growers and advisers. With the 25 focus farms and the newly introduced HYC awards, these have been targeted to scale up the results and create a community network aimed at lifting productivity.
Results from the first year of HYC research trials are currently being harvested and processed at the time of writing, however there are some early learnings and results from the previous Hyper Yielding Cereals project in Tasmania that illustrate the importance of disease management when yield potential is higher. The introduction of new fungicide technology over the last five years has increased our ability to secure a greater proportion of our yield potential in more disease prone seasons. The more extensive armoury of fungicides comes with an increased responsibility to protect our fungicides from the development of fungicide resistance and reduced sensitivity. One of the key measures we can adopt to slow down the development of fungicide resistance is to use fewer fungicides. In order to do this the industry needs the evidence that germplasm and other integrated disease management (IDM) measures can be adopted with equal profitability as multiple fungicide applications. With some growers and advisers using up to five fungicides in the 2020 season, HYC research has set out to maximise yield with the use of fewer fungicides, focusing on regions where yield potential is inherently higher.
Critical role of disease management in protecting higher yield potential in better seasons
Disease management is one of the most important components of growing high yielding cereal crops in seasons with higher yield potential or in high rainfall zone (HRZ) regions that inherently have higher yield potential. This is primarily a result of the growing season being longer, wetter and more conducive to disease development In previous Hyper Yielding Cereals research work in Tasmania, in wheat it was found that three key timings for fungicide intervention were essential to protect the upper leaves of the canopy, capture the highest yields and provide the highest economic returns; these were first node growth stage (GS) 31, flag leaf emergence (GS39) and head emergence (GS59). In barley two timings were essential; GS31 and awn tipping (GS49). In HYC 2020 research trials our objective has been to examine whether newer cultivars suitable for high yielding regions (correct phenology and standability might allow us to delay fungicide intervention and therefore use fewer fungicide applications. If a cultivar has sufficient genetic resistance to prevent disease development, it may be possible to delay fungicide application until flag leaf emergence or at least later into stem elongation (GS33-37). This has two primary benefits; firstly, it allows a much better appraisal of whether the seasonal conditions have the potential to support fungicide expenditure and secondly it means that a fungicide can be applied to more of or all of the upper canopy leaves at the same time. In those seasons where the spring progressively cuts out it means the flag leaf spray expenditure could be cut back or removed altogether. However, the industry needs good genetic resistance in our high yielding cultivars to make this a reality.
Results processed so far in HYC research in southern NSW (Table 2) and southern Victoria (Table 3) show not only the significant influence of disease management but also the large differences in genetic resistance to disease. In a season with higher yield potential and higher disease pressure (primarily Stripe rust (pt. 198 E16 A+ J+ T+ 17+), Septoria tritici blotch and lower levels of leaf rust at both locations), all wheat cultivars gave a significant yield response to fungicide application, but where cultivars had greater genetic resistance there was no statistical yield difference between a single unit of fungicide (where the flag leaf spray was based on a full rate azoxystrobin/epoxiconazole mixture (Radial® 840 mL/ha) compared to where plots were kept free of disease.
Table 1. Treatment and management applied to 2020 trials.
Plant pop’n: |
| 180 seeds/m2 (150 plants/m2 target) - all three managements | ||
|---|---|---|---|---|
| Timing | Untreated | 1 fungicide unit | 4 fungicide units |
Seed treatment: | Vibrance®/Gaucho® | Vibrance/Gaucho | As per 1 F unit + Systiva® | |
Basal Fertiliser: | 21 April | 120kg MAP | 120kg MAP | 120kg MAP |
Nitrogen: | 18 June | 40kg N/ha | 40kg N/ha | 40kg N/ha |
29 July | 70kg N/ha | 70kg N/ha | 70kg N/ha | |
Total N Applied: | 122kg N/ha | 122kg N/ha | 122kg N/ha | |
Fungicide: | GS31 | --- | --- | Prosaro® |
| GS39 | --- | Amistar Xtra® | Amistar Xtra |
| GS59-61 | --- | --- | Opus® 125 |
Table 2. Influence of fungicide strategy and cultivar on wheat grain yield (t/ha) – HYC Wallendbeen, NSW. (S.trt = seed treatment)
| Varietal yield under different levels of disease management | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Untreated | 1 fungicide unit (GS39) | 4 fungicide units (S.trt, GS31,39, 61) | ||||||
Cultivar | Yield (t/ha) | Yield (t/ha) | Yield (t/ha) | ||||||
Trojan (spring) | 2.28 | n | 7.55 | hij | 8.13 | efg | |||
Scepter (spring)* | 7.07 | kl | 8.60 | d | 8.55 | de | |||
Nighthawk (facultative) | 7.98 | gh | 8.47 | def | 8.54 | de | |||
Annapurna (winter) | 9.69 | c | 10.22 | b | 10.46 | ab | |||
RGT Accroc (winter) | 9.72 | c | 10.86 | a | 10.83 | a | |||
Beckom (spring) | 7.75 | ghi | 8.46 | def | 8.66 | d | |||
Catapult (spring) | 6.06 | m | 7.84 | ghi | 8.46 | def | |||
EGA Gregory (spring) | 6.75 | l | 7.15 | jkl | 7.40 | ijk | |||
Coolah (spring) | 7.26 | jk | 8.07 | fg | 8.75 | d | |||
DS Bennett (winter) | 5.68 | m | 8.75 | d | 9.48 | c | |||
|
|
|
| ||||||
LSD Cultivar x Man. p=0.05 | 0.45 t/ha | P val | <0.001 | ||||||
* Scepter was unaffected by wheat powdery mildew at this site.
Winter = winter wheat, spring = spring wheat, Facultative – can be grown as winter or spring wheat (depending on sowing date).
Yield figures followed by the same letter are not considered to be statistically different (p=0.05).
Table 3. Influence of fungicide strategy and cultivar on wheat grain yield (t/ha) – HYC Gnarwarre, VIC (Results with the same letter after them do not significantly differ. S.trt = Seed treatment)
| Varietal yield under different levels of disease management | |||||
|---|---|---|---|---|---|---|
| Untreated | 1 Fungicide unit (GS39) | 4 Fungicide units(S.trt, GS31,39, 61) | |||
Cultivar | Yield t/ha | Yield t/ha | Yield t/ha | |||
Trojan (spring) | 2.14 | p | 2.90 | o | 8.97 | d-g |
Scepter (spring) | 5.82 | n | 7.87 | jkl | 8.78 | efg |
Nighthawk (facultative) | 7.21 | m | 7.60 | lm | 8.11 | jk |
Annapurna (winter) | 8.30 | hij | 8.97 | d-g | 9.23 | b-e |
RGT Accroc (winter) | 7.85 | jkl | 9.13 | c-f | 9.58 | abc |
RGT Calabro (winter) | 7.67 | klm | 8.63 | gh | 8.95 | efg |
SFR 86-090 (winter) | 5.94 | n | 9.15 | c-f | 9.82 | a |
Tabasco (winter) | 7.67 | klm | 7.81 | kl | 8.11 | ijk |
SF Adagio (winter) | 8.71 | fgh | 9.67 | ab | 9.44 | a-d |
SQP Revenue (winter) | 5.71 | n | 7.92 | jkl | 8.58 | ghi |
| ||||||
LSD Cultivar x Man. p=0.05 | 0.47 t/ha | P val | >0.001 | |||
Winter = winter wheat, spring = spring wheat, Facultative – can be grown as winter or spring wheat (depending on sowing date).
Yield figures followed by the same letter are not considered to be statistically different (p=0.05).
Plot yields: To compensate for edge effect in the outside rows of the plot, yields are calculated on plot area that is larger than the area occupied by the plot itself (approximately half the plot gap either side (0.225m) is added to the plot width (1.575m) giving a total width of 2.025m. All provisional results have been analysed through ARM software with further analysis when the final results are released.
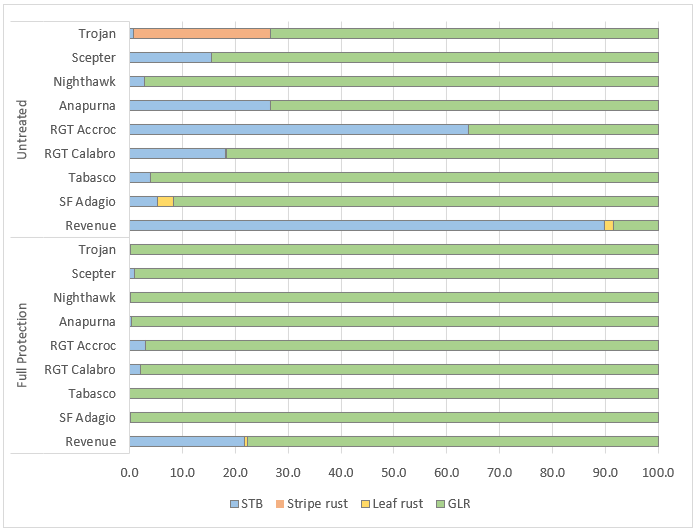
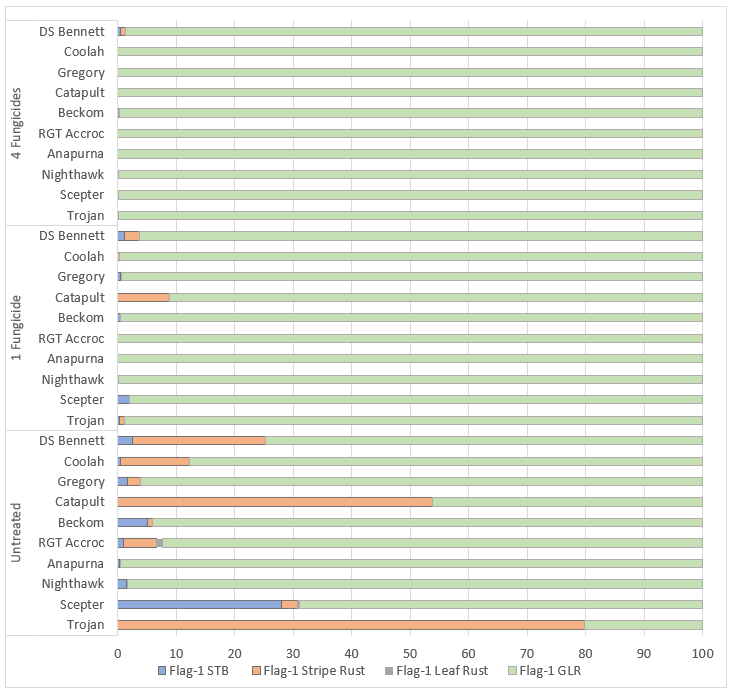
2020 was a high disease pressure year. The yield data was reflective of the level of disease, diseases present, and variety interactions experienced. At Gnarwarre in Victoria disease infection occurred much earlier in stem elongation and in some cultivars, there was considerable infection in the lower canopy (Flag-3) by the time the crop had reached flag leaf emergence (Figure 1). At Wallendbeen in NSW disease infection occurred later in stem elongation with full effects not apparent until grain fill (Figure 2).
Figure 1. Influence of fungicide strategy and cultivar on disease severity on flag-3 (% Leaf area infected & %Green Leaf retention (GLR)), 9th September (GS39 – Flag leaf emergence on the main stem) – Gnarwarre, VIC.
Figure 2. Influence of fungicide strategy and cultivar on disease severity on flag-1 (% Leaf area infected & Green Leaf retention (GLR)), 3rd November (GS75-80 – Grain fill) – Wallendbeen, NSW.
So how can we maximise productivity and minimise fungicide resistance development in seasons of high disease pressure?
Firstly, we need to know which are the most problematic pathogens for resistance development. While it’s advisable to adopt integrated disease management (IDM) principles for all diseases, some pathogens are more problematic than others. In Australia, the more problematic pathogens for resistance are powdery mildew in wheat (WPM) and barley (BPM), net blotches in barley (both spot and net form) and Septoria tritici blotch (STB) in wheat (Table 4). In addition, the risk of resistance development in these pathogens varies with fungicide mode of action.
- Group 11 QoIs (strobilurins) are at the highest risk of pathogen resistance development, particularly the pathogens responsible for Septoria tritici blotch (STB) in wheat and powdery mildew. Note at present in Australia it’s the wheat powdery mildew pathogen that is affected; globally both WPM and STB pathogens have resistance affecting QoI performance.
- Group 7 SDHIs are at moderate to high risk of resistance development in the pathogen with evidence in New Zealand and Europe of pathogen shifts in sensitivity to Ramularia leaf spot in barley and net blotch and STB in Europe. Net blotch pathogens are currently our biggest issue in Australia.
- Group 3 DMIs Demethylase Inhibitors (DMIs – triazoles) are generally considered at low to moderate risk, however recent developments in WA in the net blotch pathogen have challenged this view.
Table 4. Fungicide resistance and reduced sensitivity cases identified in Australian broad acre grains crops.
Disease | Pathogen | Fungicide group | Compounds affected | Region | Industry implications |
|---|---|---|---|---|---|
Barley powdery mildew | Blumeria graminis f.sp. hordei | 3 (DMI) | Tebuconazole, propiconazole, flutriafol | Qld, NSW, Vic, Tas, WA | Field resistance to some Group 3 DMI fungicides |
Wheat powdery mildew | Blumeria graminis f.sp. tritici | 3 (DMI) | None | NSW, Vic, Tas | This is a gateway mutation. It does not reduce the efficacy of the fungicide but is the first step towards resistance evolving. |
11 (QoI) | All group 11 | Vic, Tas, SA | Field resistance to all Group 11 fungicides | ||
Barley net-form of net blotch | Pyrenophora teres f.sp. teres | 3 (DMI) | Tebuconazole, propiconazole, | WA | Reduced sensitivity that does not cause field failure |
7 (SDHI) | Fluxapyroxad, | SA | Reduced sensitivity or resistance depending on the frequency population. | ||
Barley spot-form of net blotch | Pyrenophora teres f.sp. maculata | 3 (DMI) | Tebuconazole, epoxiconazole, propiconazole | WA | Field resistance to old generation Group 3 fungicides |
| 7 (SDHI) | Fluxapyroxad, | WA | Reduced sensitivity identified in 2020 | |
Wheat septoria leaf blotch | Zymoseptoria tritici | 3 (DMI) | Tebuconazole, flutriafol, propiconazole, cyproconazole, triadimenol | NSW, Vic, SA, Tas | Reduced sensitivity that does not cause complete field failure |
Table 3 definitions
Reduced sensitivity: Fungi are considered as having reduced sensitivity to a fungicide when a fungicide application does not work optimally but does not completely fail. In most cases, this would be related to small reductions in product performance which may not be noticeable at the field level. In some cases, growers may find that they need to use increased rates of the fungicide to obtain the previous level of control. Reduced sensitivity needs to be confirmed through specialised laboratory testing.
Resistant: Resistance occurs when the fungicide fails to provide an acceptable level of control of the target pathogen in the field at full label rates. Resistance needs to be confirmed with laboratory testing and be clearly linked with an unacceptable loss of disease control when using the fungicide in the field at full label rates.
Where the cultivar’s susceptibility to disease prevents delaying fungicide application until flag leaf (or later in stem elongation) and earlier fungicide intervention is needed (e.g. GS31) to secure the higher yield potential, it’s important that we adhere to sound anti resistance measures. These include avoiding repeated use of the same active ingredients/products and in the case of the newer Group 11 QoI (strobilurins) and Group 7 SDHIs, also avoid repeating the same mode of action. This is frequently easier said than done in longer season scenarios, since many of the fungicides with better efficacy are also important co-formulation partners in fungicide mixtures carrying two modes of action. However, focussing on the key physiological timings that protect the upper canopy leaves will ensure that the number of applications is not excessive, usually no more than two applications or three at most is sufficient with the most susceptible scenarios.
Anti-resistance measures when using fungicides as part of an integrated disease management (IDM) strategy
- With wheat and barley crops where two to three applications of fungicide are applied, avoid repeat applications of the same product/active ingredient and where possible also avoid the same mode of action in the same crop. This is particularly important when using Group 11 QoI (strobilurins) and Group 7 SDHIs, which preferably would only be used once in a growing season
- Avoid using the seed treatment fluxapyroxad (Systiva®) in successive years in barley and rotate with foliar fungicides of a different mode of action during the season
- Avoid applying the same DMI (triazole) Group 3 fungicide twice in a row, irrespective of whether the DMI is applied alone or as a mixture with another mode of action
- Avoid the use of tebuconazole alone and flutriafol for STB pathogen control, as these Group 3 DMIs are more affected by reduced sensitivity strains than other DMIs
- Group 3 DMIs (for example; triazoles e.g. epoxiconazole (Opus®) or triazole mixtures (e.g. prothioconazole and tebuconazole (Prosaro®)) used alone are best reserved for less important spray timings, or in situations where disease pressure is low in higher yielding scenarios
- With SDHI seed treatments such as fluxapyroxad (Systiva®) or QoI fungicides used in-furrow such as Uniform® containing azoxystrobin, consider foliar fungicide follow ups which have a different mode of action, and therefore, avoiding if possible, a second application of SDHI or QoI fungicides.
Influence of fungicide rate
Growers and agronomists frequently ask the question whether dose rates have an impact on how likely fungicide resistance is to evolve. Resistance comes in many forms and trying to manipulate rates with fungicides should not be seen as the core resistance management strategy. The reality is that using the most appropriate rate for effective disease control is the best strategy for managing resistance. Label rates have been developed to provide robust and reliable control of the target disease.
In many cases the full label rate is the most appropriate rate for control. However, for some diseases, the lower rate from the label range of a fungicide can be used in conjunction with a crop variety that has a good disease resistance rating because disease pressure will be lower. Contrary to what happens with herbicides, there is evidence that by using a higher rate than necessary increases the risk of resistance, as removing more sensitive individuals from the population provides more opportunity for resistant individuals to dominate the population and hence be the main strain that colonises the plant. This is particularly the case with Group 11 QoIs and Group 7 SDHIs fungicides.
Clearly, the best way to avoid fungicide resistance is not to use fungicides! However, in high disease pressure regions, this would be an unprofitable decision. When a cultivar’s genetic resistance breaks down or is incomplete, it is imperative that growers and advisers have access to a diverse range of effective fungicides (in terms of mode of action) for controlling the disease. Hence, we need to protect their longevity. In order to protect them, one of the most effective measures is to minimise the number of fungicide applications applied during the season. Therefore, consider all aspects of an Integrated Disease Management (IDM) strategy including selection of crop varieties with higher levels of genetic resistance to key diseases when putting your cropping plans together at the start of the season.
Principle components of IDM
Rotations– where possible avoid high risk rotations for disease, for example, barley on barley or wheat on wheat.
Seed hygiene – minimise the use of seed from paddocks where there were high levels of disease that could be seedborne (e.g. Ramularia, net form net blotch).
Use cultivars with higher resistance ratings particularly when sowing early. Where this is not possible delay the sowing of the most susceptible cultivars to reduce disease pressure where the phenology of the cultivar is adapted to the later development window.
Cultural control such as stubble management, where disease risks are high and the penalties for stubble removal are not as high.
Grazing early sown cereal crops up to GS30 to reduce disease pressure.
AFREN (Australian Fungicide Resistance Extension Network)
The Australian Fungicide Resistance Extension Network (AFREN) was established to develop and deliver fungicide resistance resources for grains growers and advisers across the country. It brings together regional plant pathologists, fungicide resistance experts and communications and extension specialists.
AFREN wants to equip growers with the knowledge and understanding that they need to reduce the development and manage the impacts of fungicide resistance in Australian grains crops.
As members of AFREN, the authors of this paper are keen to hear if you believe you are encountering reduced sensitivity or resistance in your broad acre crops.
Investment acknowledgement
FAR Australia gratefully acknowledges the investment of the Grains Research and Development Corporation (GRDC) for the AFREN and Hyper Yielding Crops Project which are both national initiatives.
Collaborating partners acknowledgement
FAR Australia gratefully acknowledges the support of all of its research and extension partners in Hyper Yielding Crops project. These are CSIRO, the Department of Primary Industries and Regional Development (DPIRD) in WA, SA Research and Development Institute (SARDI), Brill Ag, Southern Farming Systems (SFS), Techcrop, the Centre for eResearch and Digital Innovation (CeRDI) at Federation University Australia, MacKillop Farm Management Group (MFMG), Riverine Plains Inc and Stirling to Coast Farmers.
We would also like to acknowledge the work of our co-workers and collaborators in AFREN, in particular Dr Kylie Ireland and Dr Fran Lopez from the Centre for Crop and Disease Management (CCDM).
For more information on AFREN and fungicide resistance – Contact: Dr Kylie Ireland
(08) 9266 3541; afren@curtin.edu.au or nick.poole@faraustralia.com.au
Contact details for Hyper Yielding project research in NSW
Name: Nick Poole and Tom Price (FAR Australia), Kenton Porker (SARDI) and Rohan Brill (Brill Ag)
Business Address: Shed 2/63 Holder Rd, Bannockburn, Victoria 3331
Phone: 08 5266 1290 / 0499 888 066
Email: nick.poole@faraustralia.com.au, tom.price@faraustralia.com.au, Kenton.Porker@sa.gov.au, rohan@brillag.com.au
® Registered trademark
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994
GRDC Project Code: FAR2004-002SAX, FAR00003, CUR1905-001SAX,
Was this page helpful?
YOUR FEEDBACK