Nitrogen loss pathways. How much N is lost when urea is not mechanically incorporated after application?
Author: Graeme Schwenke, NSW DPI | Date: 17 Feb 2021
Take home messages
- If surface-applied urea is not incorporated into the soil, some of the nitrogen (N) may be lost via volatilisation of ammonia (NH3) gas from the soil to the atmosphere. Runoff of dissolved urea soon after fertiliser application can also occur if intense rainfall exceeds infiltration
- Volatilisation of broadcast urea from Australian cropping soils average 11.7% of applied N (range: 5.4–27%) from fallow soils, and 8.1% (range: 0–29%) in-crop. Field measurements of volatilised N in the grains industry have been sparse and sporadic. Globally, urea volatilisation loss averages 14.5% of applied N (includes crop, pasture, forest). Losses of up to 64% of applied N have been reported
- Loss of N via volatilisation is driven by soil properties, weather conditions, and stubble/crop cover. A simple on-line model is available to estimate NH3 volatilisation from urea applied to moist soils. Inputs required are soil pH, clay%, fertiliser rate/placement, rainfall, and crop growth stage. More field measurements are required to better validate its use across the many soils of the Australian grains industry under various soil moisture, crop/stubble cover and weather conditions
- Ammonia volatilisation loss will be low when urea is broadcast onto dry, clay soil under non-humid, non-windy conditions followed within a few days of application by sufficient rainfall to move the urea/ammonium into the soil. In contrast, NH3 loss will be higher when urea is applied to wet soil followed by dry, windy conditions with little or no follow-up rainfall.
Nitrogen pathways
The nitrogen (N) cycle in the soil, water and air is complex (Figure 1). There are many solid, solution and gaseous forms of N that are interconnected by a variety of processes—some biologically-driven others purely chemical. What diagrams like Figure 1 do not show, are the relative amounts of N present in the different forms and the different rates of change from one form to another. In many situations, only some of these pathways are relevant, with the actual conditions dictating which will dominate. For example, N loss to the air via denitrification can be a major pathway for loss of nitrate-N in clay soils if they become anaerobic due to waterlogging, but denitrification is typically minimal otherwise. Likewise, soil erosion can lead to catastrophic losses of both organic and inorganic N forms in extreme situations, but should be minor under more normal weather conditions and well-managed (minimised) surface water flow.
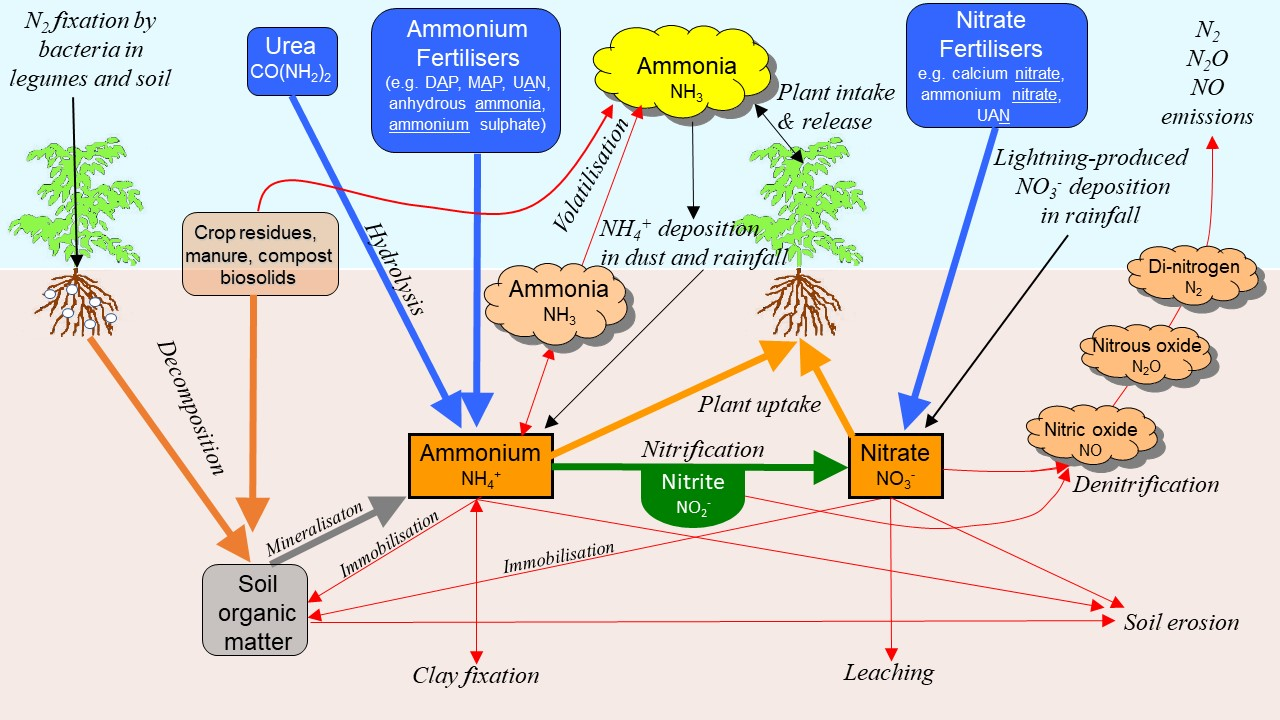 Figure 1. Major pathways and forms in the nitrogen cycle in soil, water and air.
Figure 1. Major pathways and forms in the nitrogen cycle in soil, water and air.
The specific situation considered here is the fate of urea fertiliser applied to the soil surface but not mechanically incorporated. What happens next depends on the soil properties, the weather, stubble/crop cover and the soil surface condition.
Urea dissolves
Urea is highly soluble (1079 g/L at 20oC) so broadcast granules dissolve on contact with dew, rain, wet soil, wet plant/crop residue surfaces or even a humid atmosphere. Urea is hygroscopic, meaning it will absorb moisture from the air depending on the ambient relative humidity and air temperature. If the critical relative humidity is exceeded, urea will quickly dissolve. As temperature increases from 5 to 35°C, the critical relative humidity required for urea to dissolve decreases from 83.9% to 72.6%. Humidity within furrows, surface soil cracks and indentations, stubble cover and crop canopies may be greater than in the bulk atmosphere and therefore dissolve urea falling into these microsites. In contrast, urea scattered on bare, flat soil surfaces during cold, low-humidity conditions may remain undissolved for up to several weeks (Schwenke et al., 2014).
One possible scenario where broadcasting urea may lead to immediate N loss from the target paddock is where the urea is applied to a soil profile that is already at field capacity and is then immediately followed by high-intensity rainfall. If the rainfall intensity exceeds the rate of water infiltration into the soil, the dissolved urea may be carried off the target field in surface water flow, or at least be concentrated into the furrows and downslope paddock areas.
Within the soil, dissolved urea moves easily via diffusion and mass flow as it has no ionic charge. Therefore, the best chance to move applied urea-N into the soil via rainfall or irrigation is as soon as possible after broadcasting while it is still in the urea form. In irrigated cropping, urea is commonly broadcast directly ahead of irrigation or water-run in irrigation water (fertigation).
Urea hydrolysis
Once dissolved, urea chemically decomposes via hydrolysis (Figure 2). Hydrolysis is a chemical process in which a molecule of water ruptures another chemical bond—in this case the two carbon-nitrogen bonds in a urea molecule. Urea hydrolysis is accelerated (catalysed) by urease, an enzyme naturally found in numerous bacteria, fungi, algae, plants, some invertebrates and in bulk soils. The activity of urease tends to decrease with soil depth as does biological activity. If the enzyme were not present, the reaction would take years rather than minutes/hours, but ureases are ubiquitous in agricultural landscapes. In a warm, moist soil urea hydrolysis normally takes 3–7 days. Volatilisation of N does not occur until urea is hydrolysed so slowing or inhibiting the process allows more time for rainfall to move urea into the soil before ammonium is produced.
During hydrolysis, dissolved urea reacts with water (H2O) and consumes H+ ions from the soil solution to form bicarbonate (HCO3-) and ammonium (NH4+) ions (Figure 2). The HCO3- then consumes more H+ ions from the soil to form carbon dioxide (CO2) and H2O. Where the urea is placed at the soil surface, the CO2 gas readily escapes and the HCO3- continues to be lost.
The consumption of H+ ions from the soil in both the hydrolysis and the HCO3- breakdown reactions causes an increase in pH (more alkaline) at the reaction sites—how much of an increase depends on the soil’s pH and its pH-buffering capacity. Soils with a low buffering capacity will show a greater pH increase during urea hydrolysis than soils with a high capacity. Soil pH buffering capacity is mainly determined by its clay minerals (type and amount) and organic matter content.
When the pH in the hydrolysis reaction zone exceeds 8.2, the breakdown of HCO3- may instead produce carbonate (CO32-) ions which slows the net increase in pH. The final pH reached has a major influence on the subsequent loss of NH3 gas via volatilisation (see next section).
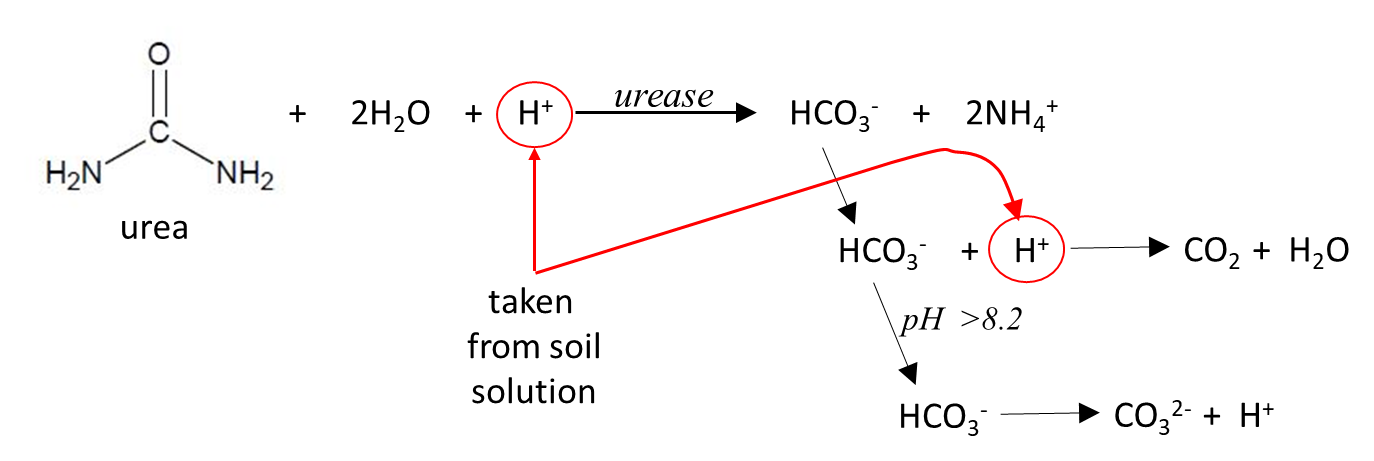 Figure 2. The urea hydrolysis process (Kissel and Cabrera, 2005).
Figure 2. The urea hydrolysis process (Kissel and Cabrera, 2005).
How and where the urea is applied will also influence the scale of the pH changes associated with hydrolysis. When urea is applied upon or within the soil, there will be a strong gradient from a very high pH at the hydrolysis reaction site, to the bulk soil pH, with an intermediate zone where pH is mediated to some degree by the interaction with the soil. This gradient occurs over millimetres in the soil. Sherlock et al. (1986) showed that soil pH around a surface-applied urea granule ranged from 9 (at the edge of the granule) to 6 (bulk soil) over a distance of just 14 mm.
Application of urea in bands means that the pH change due to hydrolysis will impact on a smaller volume of soil and therefore lead to a greater, more prolonged increase in pH at the reaction zone. A laboratory study using an acidic clay loam soil in Canada by Rochette et al. (2009), found greater NH3 volatilisation associated with shallow (3–5 cm) banding than from surface broadcasting—due to greater pH change during hydrolysis in the banded treatments.
Applying urea at higher rates will also lead to greater pH increases in this zone. Where broadcast urea is incorporated, the change in pH at the reaction zones is likely to be less as the urea is in contact with a greater volume of soil and its concentration in the reaction zone is much less.
The rate of urea hydrolysis in soils is affected by (a) concentration/activity of urease, (b) temperature, (c) water content and (d) pH. After surface spreading urea, there is typically a ‘lag’ in the onset of hydrolysis of up to several days after urea has dissolved. This lag is thought to be due to the time it takes for the dissolved urea to diffuse down into the soil and contact the urease catalyst. Therefore, hydrolysis is quicker if the urea is applied directly as a solution instead of granules. While urease enzymes are found in almost all soil/plant/stubble environments, rates of hydrolysis can be affected by the actual amount of urease present. Urease catalysis can be inhibited by very high concentrations of dissolved urea, which can be of benefit as it gives more time for rainfall to occur and move the dissolved urea lower into the soil.
Warmer temperatures increase the rate of hydrolysis—a 4-fold increase between 5oC and 25oC. Hydrolysis at 27oC will be 90% complete after 3 days but will take 9 days at 2oC. Since hydrolysis requires available water, the hydrolysis reaction slows down in dry soil conditions (below plant wilting point) and stops altogether when soils are air-dry. It is also more rapid when the urea is incorporated, as soil moisture is typically greater with increasing soil depth from the surface. Hydrolysis is typically optimised in a pH range from 8–9, but optimum pH effects vary between soil types.
Ammonium pathways
Once urea has been hydrolysed to form ammonium (NH4+) in the soil solution, it follows one or more competing pathways, each with specific rate-determining factors (Figure 3). Only in very dry soils will NH4+ persist unchanged. Nitrification, plant uptake and microbial immobilisation generally remove most of the NH4+ over time, with rates depending primarily on soil moisture and plant growth demand. Most plant species prefer to take up nitrogen in the nitrate form, but plants also take up NH4+ for growth.
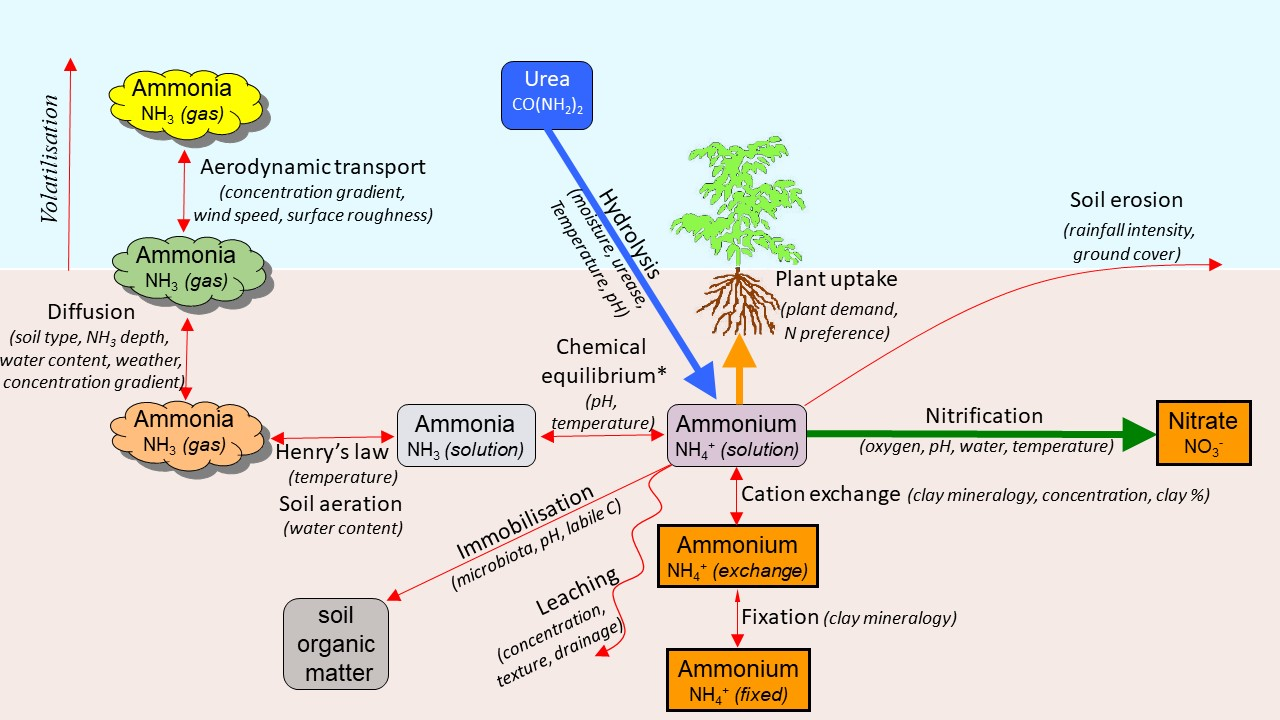 Figure 3. Pathways and rate-determining factors for the transformation of ammonium N formed from urea hydrolysis in the soil (adapted from Cichota and Snow (2012)).
Figure 3. Pathways and rate-determining factors for the transformation of ammonium N formed from urea hydrolysis in the soil (adapted from Cichota and Snow (2012)).
Ammonium adsorption on the soil’s cation exchange sites greatly reduces the amount of NH4+ in the soil solution. This is why many researchers have found that soils with a CEC >25–30 cmol+/kg have vastly reduced losses via NH3 volatilisation. Soil CEC is closely related to soil clay content although there is some variation between different clay minerals.
Ammonium can be rendered unavailable to uptake/nitrification by;
- Fixation within clay minerals—especially illite, vermiculite and to a lesser extent montmorillonite, or
- Microbial immobilisation during the decomposition of organic materials with high C/N ratios, such as cereal crop roots, straw or stubble.
Both fixation and immobilisation can be regarded as temporary losses, as the captured NH4+ is released back into the soil solution over time.
Ammonium is rarely observed to leach in soils as its positive charge bonds it to cation exchange surfaces on clay minerals and organic matter. However, where concentrations of NH4+ are very high, such as under urine patches or in high N-rate fertiliser bands, or if applied to sandy soils, the soil’s exchange capacity may be exceeded and downward movement with water can occur.
Ammonium in surface soil can also be lost via episodic wind and water erosion events.
Ammonia volatilisation
When ammonium is present in the soil solution, a series of chemical reactions and soil physical properties can lead to it being volatilised as NH3 (Figure 3). Principal among this chain of processes is the equilibrium between NH4+ and NH3 in solution (Figure 4).
Figure 4. The equilibrium between ammonium (NH4+) and ammonia (NH3)
This equilibrium is strongly governed by pH and temperature (Figure 5). This is where the changes in pH generated by hydrolysis become important to determining the amount of N loss.
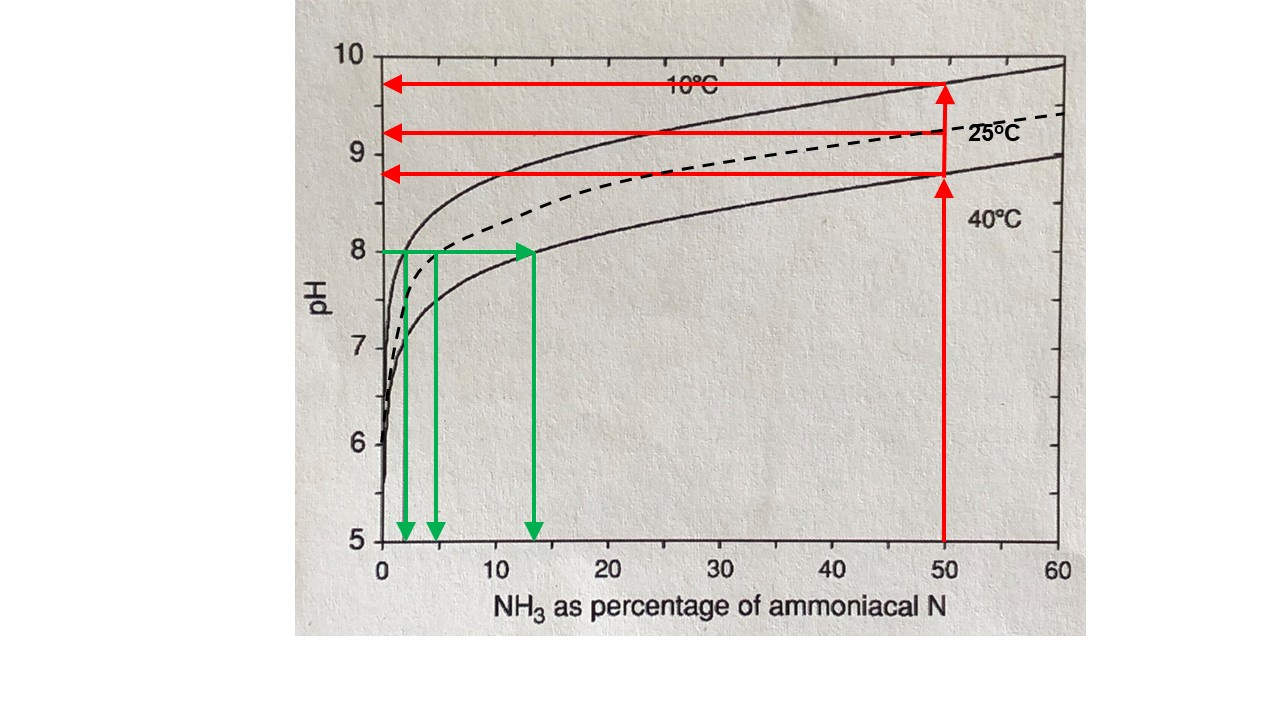
Figure 5. The percentage of total ammoniacal N (sum of NH4+ and NH3) made up by NH3, as affected by solution pH and temperature (Kissel and Cabrera, 2005). Green arrows show difference in percentages according to temperature at pH = 8. Red arrows show pH required at different temperatures for NH3 to be 50% of total ammoniacal N
As pH increases (i.e. becomes more alkaline), more of the total ammoniacal N (sum of NH4+ and NH3) will be present as NH3. For example, at a pH of 8, the proportion present as NH3 will be 2% at 10oC, 5% at 25oC and 13% at 40oC (follow the green arrows on Figure 5). The pH where the proportions of NH4+ and NH3 are equal (known as the pKa value) will be 9.75 at 10oC, 9.25 at 25oC and 8.8 at 40oC (follow the red arrows on Figure 5). These high pHs can occur naturally in highly alkaline soils but will also develop in the urea hydrolysis reaction zone of acidic-neutral soils. When NH3 is produced from NH4+, a H+ ion is produced (Figure 4). In alkaline soils, this H+ can be neutralised (buffered) by carbonate ions (CO32-) in the soil solution and the reaction can continue. However, in acidic or poorly buffered soils, the additional H+ will cause pH to decrease and the equilibrium between ammonium and ammonia will favour ammonium, and reduce the potential for volatilisation loss.
Ammonium and ammonia in solution can move within the soil as water moves either;
- Upwards via capillary rise and evaporation, or
- Downwards in percolating rainfall or irrigation water.
Ammonia/ammonium also diffuse outwards from concentrated sources such as granules or bands.
The partitioning between NH3 present in solution and NH3 present in air contacting that solution is described by an equilibrium constant for NH3 in Henry’s law (at a constant temperature, the amount of a gas that dissolves in a liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid). Then, as temperature increases, more of the NH3 will be present as a gas because the soil solution can hold less NH3 gas than in colder solutions. At 40oC the soil air will hold three times as much NH3 gas than at 10oC. This equilibrium is also affected by the soil’s aeration which will limit the contact of the solution with the air in the soil.
The next phase within the volatilisation process is the movement of gaseous NH3 from the soil to the bulk atmosphere. For the NH3 gas to escape, it must first move via diffusion or evaporation through soil pores to the surface. Soil structure and texture will influence the porosity-driven pathway to the surface. Along the way, contact of NH3 gas with soil moisture that is low in NH3 will cause it to go from gas form back into solution. Ammonia gas moving to areas of lower pH will also affect the NH3/NH4+ equilibrium and thus potentially move back to the NH4+ form where it can be held on the soil’s cation exchange surfaces. Ammonia gas can also be directly adsorbed to dry soil mineral and organic matter surfaces, so soils with greater surface area (finer texture = higher clay content) will adsorb more. These are all reasons why banding or incorporation broadcast urea into the soil typically reduce volatilisation losses, particularly in acidic soils.
Ammonia gas produced at or near the soil surface is said to have volatilised once it has been transported into the bulk atmosphere. This process is driven by the concentration gradient between the NH3 gas in the soil and in the air directly above the soil (boundary layer). Where the concentration of NH3 in the soil air is greater than the concentration in the boundary layer air, the NH3 gas will move out of the soil and volatilise. This is why the rate of NH3 volatilisation is often linked to wind speed, surface roughness and air turbulence. These factors regulate the removal of NH3-rich air away from the boundary layer at the soil surface, replacing it with ‘fresh’ air of lower NH3 concentration (creating a higher concentration difference compared to that emitted from the soil). The presence of a crop can greatly reduce the wind speed at the soil surface and therefore markedly reduce volatilisation losses.
Ammonia transport and deposition
In a cropping scenario, some of the NH3 volatilised from the soil surface may be absorbed directly into or onto plant leaves before it escapes above the canopy. How much is absorbed by the crop depends on the difference in concentration of NH3 in the air compared to that in the plant’s sub-stomatal cavities. Field measurements of NH3 volatilisation are typically done above the plant canopy and so represent the net loss after the crop has absorbed what it can.
Depending on wind and air currents, volatilised NH3 can be transported just meters away, or up to a hundred kilometres away from the source. However, NH3 is readily absorbed onto dust particles and raindrops resulting in it commonly being deposited back to earth relatively close to the source (Figure 1). Ammonia in the air can also react with acidic air-borne chemicals to form extremely small particulates (P2.5 = <2.5 µm diameter) that can persist in the air for weeks and contribute to atmospheric haze and aggravate health issues in humans. Transport of these NH4+-based particulates can occur over much larger distances, up to and exceeding 1000 kilometres. Deposition will be determined by ecosystem type and weather conditions.
Field measurements of ammonia volatilisation losses from Australian cropping soils
Measuring NH3 volatilisation in field situations is best done in open-air plots that do not restrict the wind flow across the treated area. The treated area, commonly a large circular plot, must be spatially separated from other treated areas to prevent confounding concentration gradient effects. Normal agronomy-trial plots or small chambers are generally not suitable. Ammonia volatilisation from broadcast urea typically begins within a day or two of spreading, peaks during the next few days, then gradually diminishes over the following 1–2 weeks as the available NH3 in the soil is either lost or depleted via other NH4+ pathways. Measurements continue for up to 1 month after fertiliser application to capture all NH3 emitted and cover all variations in weather conditions. Indirect measurements of inorganic N concentrations in soil over time are unreliable as they do not account for plant uptake, soil microbial immobilisation or other N losses such as leaching or denitrification.
Field-measured volatilisation losses from urea applied to Australian cropping soils averaged 11.7% of applied N (range: 5.4–27%) from fallow soils, and 8.1% (range: 0–29%) of N applied in-crop. Globally, NH3 volatilisation losses from urea use average 14.5% of the N applied (includes pastures, horticulture, forestry and irrigated cropping in all climatic zones), with losses of up to 64% reported (Ma et al., 2020). Australian measurements were made in north-west NSW (Schwenke et al., 2014), southern NSW (Griffith) (Bacon and Freney, 1989), western Victoria (Wimmera) (Turner et al., 2010; Turner et al., 2012) and Western Australia (Merredin, Regans Ford) (Fillery and Khimashia, 2016). Clearly, field measurements across the Australian grains industry have been sparse and sporadic.
Ammonia volatilisation has also been measured for other N-fertilisers and manures, and in other Australian industries, including pastures and sugarcane. In the northern NSW study, losses from broadcast urea averaged 27% when applied to two grass pastures as much of the urea was held up by the thatch and did not contact the soil—a situation that may be similar to thick stubble cover. Nitrogen losses from ammonium sulfate were less than half the losses from urea at 2 pasture sites and 5 of 8 fallow paddocks on non-calcareous soils but were higher than urea (19–34% N loss) from fallowed soils naturally containing more than 10% calcium carbonate (Schwenke 2014). This is because of a specific chemical reaction between ammonium sulfate and calcium carbonate that drives the formation of unstable ammonium carbonate. Urea is not affected in the same way.
Crop residue effects on NH3 volatilisation have had little attention in Australian research, but in general terms the trend from global research is for the presence of residues to increase NH3 emissions compared to bare soil (Ma et al., 2020). This could be due to increased urease activity, greater NH4+ availability from decomposition, greater localised humidity, and even preventing urea granules reaching the soil surface. In one of the paddocks measured by Bacon and Freney (1989), high NH3 volatilisation from urea spread at sowing was explained by a thick surface covering of highly alkaline ash (pH = 9.5) from burnt rice stubble. In addition to the high pH, the ash would have also saturated the cation exchange sites with basic cations preventing adsorption of NH4+. Delaying N application till later in the growing season considerably reduced this loss.
Urea can be coated with a urease inhibitor to delay the hydrolysis process and allow more time for rainfall to occur and move the more mobile urea into the soil. While there are many compounds that can inhibit the urease enzyme, the main one available for use in Australian is NBPT [N-(n-butyl) thiophosphoric triamide]. Globally, urea coated with NBPT has been shown to reduce NH3 volatilisation losses by around half, on average, when compared to uncoated urea (Ma et al. 2020).
A model for estimating urea losses via ammonia volatilisation
With so many processes and rate-determining factors affecting the volatilisation loss of NH3, it should be clear that the actual amount of N lost will depend on soil properties (especially clay type and clay%, pH, texture, structure), the soil moisture content and humidity when the urea was applied, and the weather conditions (temperature, rainfall, wind) experienced for the following days–weeks after applying it. Biophysical (mechanistic) models using detailed understanding of the physical and chemical processes in soil described in the previous sections have been developed, but these require many details and specific soil properties that are not routinely available. Models based on observed relationships between measured NH3 volatilisation and major influencing factors are more suitable for use by growers, advisors and most researchers.
Fillery and Khimashia (2015) published a simple model to predict NH3 volatilisation losses from fertiliser applied to moist soils. Their model starts with a maximum potential loss figure (65%) which is then ‘discounted’ according to the effects of input factors including clay content, soil pH, fertiliser rate, rainfall in the week after application, presence and growth stage of a crop canopy, and the placement of the fertiliser. The starting figure and discount factor algorithms were not derived from Australian research, but the model did a reasonable job of predicting the losses measured in the previous Australian studies (and some Chinese and American ones). The model is not suitable for soils that are very dry at the time of application as this delays the soil processes involved. Further validation is needed using field measurements on a greater range of Australian soil types and soil moisture contents at spreading. The impacts of crop residues (amount and orientation), timing of N application throughout the year, application methods/rates and subsequent weather conditions all need further research.
This model can be used via an online calculator on the BackPaddock Website.
Acknowledgements
Many thanks to Jason Condon and Mark Conyers for reviewing this paper.
References
Bacon PE and Freney JR (1989) Nitrogen loss from different tillage systems and the effect on cereal grain-yield. Fertilizer Research 20(2), 59-66.
Cichota R and Snow V (2012) Ammonia Volatilisation from Grazed Pastures: Report for Dairy Australia Project C100000293 N transformations and loss pathways Sub-project 2B: Volatilisation Final version – 24 May 2012.
Fillery IRP and Khimashia N (2016) Procedure to estimate ammonia loss after N fertiliser application to moist soil. Soil Research, -.
Kissel DE and Cabrera ML (2005) Ammonia. In 'Encyclopedia of Soils in the Environment.' Ed. D Hillel) pp. 56-64. (Elsevier Ltd)
Ma R, Zou J, Han Z, Yu K, Wu S, Li Z, Liu S, Niu S, Horwath WR, Zhu-Barker X (2020) Global soil-derived ammonia emissions from agricultural nitrogen fertilizer application: A refinement based on regional and crop-specific emission factors. Global Change Biology n/a(n/a).
Rochette P, MacDonald JD, Angers DA, Chantigny MH, Gasser M-O, Bertrand N (2009) Banding of Urea Increased Ammonia Volatilization in a Dry Acidic Soil. Journal of Environmental Quality 38(4), 1383-1390.
Schwenke GD, Manning W, Haigh BM (2014) Ammonia volatilisation from nitrogen fertilisers surface-applied to bare fallows, wheat crops and perennial-grass-based pastures on Vertosols. Soil Research 52(8), 805-821.
Sherlock RR, Black AS, Smith NP (1986) Microenvironment soil pH around broadcast urea granules and its relationship to ammonia volatilization. In 'Nitrogen Cycling in Temperate Agricultural Systems. ' (Eds PE Bacon, J Evans, RR Storrier and AC Taylor) pp. 316-326. (Australian Society of Soil Science: Riverina Branch, Wagga Wagga)
Turner DA, Edis RB, Chen D, Freney JR, Denmead OT, Christie R (2010) Determination and mitigation of ammonia loss from urea applied to winter wheat with N-(n-butyl) thiophosphorictriamide. Agriculture Ecosystems & Environment 137(3-4), 261-266
Turner DA, Edis RE, Chen D, Freney JR, Denmead OT (2012) Ammonia volatilization from nitrogen fertilizers applied to cereals in two cropping areas of southern Australia. Nutrient Cycling in Agroecosystems 93(2), 113-126
Contact details
Graeme Schwenke
NSW Department of Primary Industries
Tamworth Agricultural Institute, Calala, NSW
Ph: 0418 636 421
Email: graeme.schwenke@dpi.nsw.gov.au
Was this page helpful?
YOUR FEEDBACK
