Pre-breeding canola for heat stress tolerance – a prototype facility for large-scale screening at flowering stage
Pre-breeding canola for heat stress tolerance – a prototype facility for large-scale screening at flowering stage
Author: Sheng Chen, Katia Stefanova, Kadambot H. M. Siddique and Wallace A. Cowling. (Institute of Agriculture, The University of Western Australia) | Date: 11 Feb 2021
Key messages
- Timing of heat stress: The critical period for heat stress in canola begins one week before first flower and continues during the flowering period. Flowers subject to heat stress produce fewer pods and seeds.
- Intensity and duration of heat stress: A heat wave of three days with a maximum temperature of 32°C from midday for four hours, and an overnight minimum temperature of 22°C, is sufficient to reduce canola yield.
- Genotypic variation in heat tolerance: Genotypes vary in heat stress tolerance, based on their ability to set seed after heat stress during flowering.
- We are developing breeder-friendly methods for genetic improvement of heat tolerance in canola.
Aims
This research is developing methodology to facilitate large-scale screening of heat stress tolerance in canola at the flowering stage and will identify heat tolerant germplasm for Australian plant breeders. The new methods and heat tolerant germplasm will be transferred to canola breeders, which will accelerate the future commercial release of heat tolerant varieties. Our aim is to help Australian growers to maintain canola productivity as temperatures rise in response to climate change.
Introduction
Transient daily heat stress during flowering of canola (Brassica napus L.) is an increasing threat to grain production due to global warming. Canola production was reduced by temperatures greater than 29.5°C during flowering in Canada (Morrison and Stewart 2002) and there was a significant association between the number of days above 30°C and low canola seed yield in Australia (Dreccer et al 2018).
Research into heat stress in several Brassica species at The University of Western Australia (UWA) over the past decade has shown that heat stress caused abnormal flower morphology and reduced pod and seed set (Annisa et al 2013). We found that transient daily heat stress for three days after first open flower in canola disrupted gametogenesis, pollination, fertilisation, early embryogenesis and seed and pod development. We were careful to avoid drought stress during the heat treatments, because drought and heat stress tolerance are most likely controlled by different genes. Under well-watered conditions, heat stress did not reduce leaf stomatal conductance, fresh weight of above-ground biomass, whole plant volume and flower volume compared with the control, but significantly decreased the number of fertile pods, number of seeds per pod, and seed yield per plant (Chen et al 2019, 2020). As a result, heat stress caused a significant decrease in harvest index (Chen et al 2020).
Our results are supported by canola heat stress research in Canada. Young et al (2004) showed that transient daily heat stress of 35/18°C day/night for 7 or 14 days reduced seed yield when the stress treatment was applied at the time when 50% of plants were flowering. Koscielny et al (2018) also used transient daily heat stress of 31/14°C day/night for 14 days from one week before first open flower.
This research is to establish the critical timing, duration and intensity of daily transient heat stress treatment during the reproductive phase of B. napus from the beginning of meiosis on the main stem inflorescence through to early pod formation, based on subsequent pod and seed formation. We aim to develop large-scale heat screening technology and promote its adoption by commercial canola breeders. We will identify heat tolerant germplasm of interest to canola breeders for the breeding of future heat tolerant commercial cultivars.
Method
All experiments were conducted at UWA. Two experiments were conducted in a glasshouse and subjected to heat-stress treatments in controlled environment rooms (CERs). After the heat-stress treatments, the plants were returned to the original glasshouse bench and grown until seed maturity. Field experiments were used to screen 257 genotypes for heat stress tolerance over multiple years, where putative heat tolerant genotypes were identified and later confirmed in CERs.
CER experiment 1: temperature and duration of heat-stress treatments
The duration of the heat treatments was 3, 5 or 7 days, with a photoperiod of 16 hours day/8 hours night. The daily heat stress was mimicked with temperatures ramping up and down each day from minimum to maximum. The temperature combination for the control treatment (TC1) had 25/15°C day/night temperatures. The moderate heat treatment was 32/22°C day/night (TC2) and the high temperature treatment was 35/25°C day/night (TC3). Two additional high-temperature stress treatments at 32/15°C day/night (TC4) and 35/15°C day/night (TC5) were used to explore the recovery from heat stress under cooler nights. During heat-stress treatments, all pots were watered automatically to maintain approximately 90% field capacity.
CER experiment 2: stage of development at the start of heat treatment
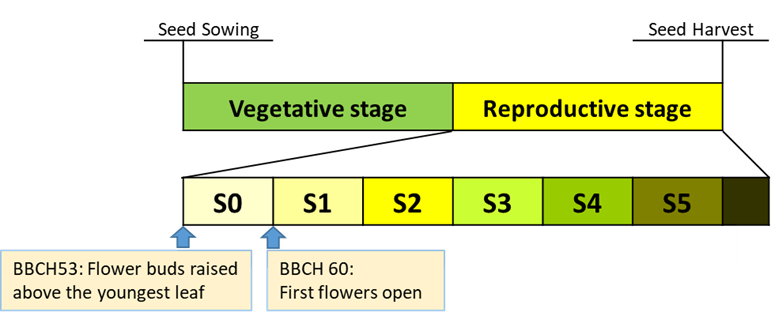
Plants were subjected to heat stress in six weekly windows S0 to S5 (Figure 1). S0 corresponds to flower buds above the youngest leaf on the main stem, and S1 represents the first open flower on the main stem (Figure 1).
Figure 1. Reproductive stage of B. napus dissected into six weekly windows: S0, 7 days before first open flower; S1, 7 days after first open flower (DAF); S2, 8–14 DAF; S3, 15–21 DAF; S4, 22–28 DAF; and S5, 29–35 DAF.
CER experimental design and statistical analysis
A randomised complete block design was used for all experiments. The treatment structure reflected a typical four-factorial experiment, with genotype (G), stage of development entering heat treatment (S), temperature regime in heat treatment (T) and duration of heat treatment (D) as factors. A linear mixed model was used for the analyses, where the blocking structure, as described above, was fitted as random and the treatment structure as fixed. The analyses were conducted using ASReml-R.
Results
Experiment 1: effect of temperature and duration of heat stress treatment at S0 and S1
There were significant main effects of genotype (G) and heat treatment (T), and G×T interactions, for most reproductive traits on the main stem including pod number (PNM), seeds per pod (SPM) and seed yield (SYM), but heat stress did not affect 100 seed weight (SWM) (Figure 2). This confirms that genetic variation exists in heat stress tolerance among genotypes in this experiment. However, the duration of heat treatment (3, 5 or 7 days) did not significantly change the PNM or SPM. Three days of heat stress is sufficient to identify genetic variation for heat stress tolerance.
Figure 2. Effect of heat-stress treatments at S0 and S1 on four agronomic traits measured on the main stem at maturity: (A) seed yield (SYM), (B) pod number (PNM), (C) seeds per pod (SPM) and (D) 100 seed weight (SWM). TC1 is the control (25/15°C), TC2 is the 32/22°C heat-stress treatment and TC3 is the 35/25°C heat-stress treatment. Bars indicate the standard error of the difference between means (SED).
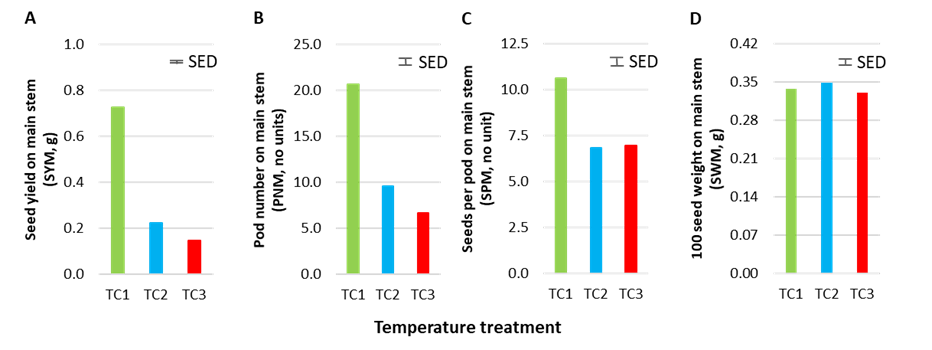
Mean SYM decreased from TC1 (0.727g), TC2 (0.224g) to TC3 (0.147g). However, heat stress did not disrupt vegetative growth of plants, which responded positively to the heat stress treatments with increases in plant height (PH) and above-ground biomass (BIO) as heat stress increased from TC1, TC2 to TC3. There were no G×T interactions for these traits; that is, genotypes behaved similarly across heat stress treatments for PH and BIO. Therefore, harvest index declined significantly from TC1, TC2 to TC3.
Experiment 2: effect of heat-stress treatments at various stages of reproduction from S0 to S5
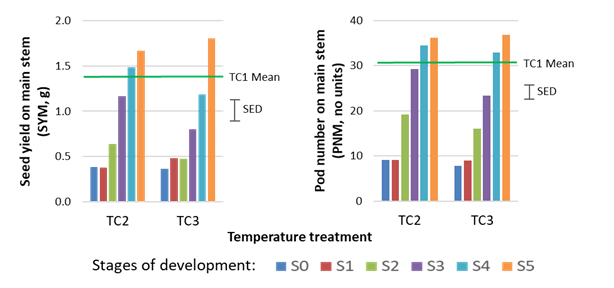
Pod and seed development on the main stem were severely reduced by heat stress at early stages of reproductive development (S0, S1, S2), but not at later stages (S3, S4, S5) (Figure 3). At S3, S4 and S5, heat stress reduced pod and seed development on the lateral branches (results not shown). Genotypes varied in their response to heat stress in both experiments 1 and 2.
SYM and PNM responded similarly when the high temperature treatment TC3 (35°C daily transient heat stress) was modified with cool nights (TC5), that is, pod and seed development on the main stem was disrupted at early stages of reproduction (S0 to S2) and did not recover with the cooler nights in TC5. In contrast, mean SYM and PNM showed recovery with cool nights in TC4 compared with TC2, indicating that warm nights in TC2 inhibited recovery in SYM and PNM from the daily temperature maximum of 32°C, relative to TC4 where nights were cooler.
The total number of reproductive nodes on the main stem was the same in all heat treatments (average 60), therefore the pod set rate was lower in TC2 (37.8%) and TC3 (34.4%) compared with the control TC1 (45.0%). There were also fewer seeds per pod on the main stem when heat stress occurred in the early stages of reproduction. Aborted seeds in pods were often small and shrivelled, and some well-developed pods had very few fully-developed seeds or none at all; however, when seeds developed in TC2 and TC3 they were of similar size (SWM) to the control TC1.
Figure 3. Effects of heat-stress applied at six growth stages (S0 to S5) on (A) seed yield on main stem (SYM), and (B) pod number on main stem (PNM). TC1 is the control (25/15°C), TC2 is the 32/22°C heat-stress treatment and TC3 is the 35/25°C heat-stress treatment. Bars indicate the standard error of the difference between means (SED).
Genotypic variation in heat tolerance in field tests
A collection of 257 B. napus lines from all over the world was screened for heat tolerance for two years under field conditions, and the putative heat-tolerant and heat-susceptible were further assessed for heat tolerance in CERs. The results showed that B. napus genotypes vary in heat stress tolerance, based on their ability to set seed after heat stress during flowering.
Figure 4. Birds-view of the prototype pre-breeding facility for canola heat-tolerance screening at UWA Field Station (Shenton Park, 2020). The core part of the facility is the two CERs, each with a heating system, cooling system, irrigation system, and ventilation system, which are all controlled centrally. The CERs are connected to two screen houses where large numbers of genotypes are grown before and after heat screening.

Conclusion
Significant genetic variation exists among canola germplasm for tolerance to heat stress. We are developing a prototype pre-breeding facility for heat stress tolerance (Figure 4) that could be incorporated into commercial canola breeding programs as part of an optimised breeding strategy for heat stress tolerance, grain yield, disease resistance and other important agronomic traits (Cowling et al 2019). We suggest that TC2 (transient daily maximum 32°C, minimum 22°C) would be suitable for large-scale screening for heat tolerance in canola, because TC2 produced wider variation among genotypes in seed yield and pod formation on the main stem than TC3. It was important to keep night temperatures warm (22°C) in TC2 to observe this genetic variation among genotypes.
The large-scale canola heat tolerance screening facility permits testing of hundreds of individual plants that are exposed to transient heat stress (TC2) for one week after first open flower and are compared with the control treatment (TC1). We identified heat tolerant genotypes by measuring pod and seed formation on the main stem at harvest. Heat tolerant plants had similar pod and seed set under heat stress as in the control.
Acknowledgments
This research was primarily supported by GRDC-funded National Brassica Germplasm Improvement Program (DAN00117, DAN00208) on canola heat tolerance research and “Improving canola heat tolerance - a coordinated multidisciplinary approach” (UWA1905-007RTX).
References
Annisa, Chen S, Turner NC, Cowling WA (2013) Genetic variation for heat tolerance during the reproductive phase in Brassica rapa. Journal of Agronomy and Crop Science 199:424–435.
Chen S, Guo Y, Sirault X, Stefanova K, Saradadevi R, Turner NC, Nelson MN, Furbank RT, Siddique KHM, Cowling WA (2019) Non-destructive phenomic tools for the prediction of heat and drought tolerance at anthesis in Brassica species. Plant Phenomics 2019:1-16.
Chen S, Stefanova K, Siddique KHM, Cowling WA (2020) Transient daily heat stress during the early reproductive phase disrupts pod and seed development in Brassica napus L. Food and Energy Security 2020; 00:e262. https://doi.org/10.1002/fes3.262
Cowling WA, Li L, Siddique KHM, Banks RG, Kinghorn BP (2019) Modeling crop breeding for global food security during climate change. Food and Energy Security 8:e00157.
Dreccer MF, Fainges J, Whish J, Ogbonnaya FC, Sadras VO (2018) Comparison of sensitive stages of wheat, barley, canola, chickpea and field pea to temperature and water stress across Australia. Agricultural and Forest Meteorology 248:275-294.
Koscielny CB, Hazebroek J, Duncan RW (2018) Phenotypic and metabolic variation among spring Brassica napus genotypes during heat stress. Crop and Pasture Science 69:284-295.
Morrison MJ, Stewart DW (2002) Heat stress during flowering in summer Brassica. Crop Science 42:797-803.
Young LW, Wilen RW, Bonham-Smith PC (2004) High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility, induces fruit abortion, and disrupts seed production. Journal of Experimental Botany 55:485-495.
Contact details
Sheng Chen
The UWA Institute of Agriculture, The University of Western Australia
35 Stirling Hwy, Perth, WA 6000, Australia
Ph: 0423238218
Email: sheng.chen@uwa.edu.au
GRDC Project Code: DAN00107, DAN00208, UWA1905-007RTX,
