Principles and strategies for managing soil acidity
Principles and strategies for managing soil acidity
Key messages
- Liming acidic, Al-toxic soil results in greater root growth and nutrient uptake.
- Dissolution of lime and increase in pH of an acidic soil are enhanced in soil layers with high soil water content.
- Very fine lime (0.05mm) reacts rapidly in soil, even where water content is low.
- When deep slotting lime, the proximity of wheat rows to a limed slot is the major factor influencing greater wheat growth.
Introduction
Soil acidity increases the concentration of dissolved aluminium (Al) in soil solution (Figure 1). Although the amount and forms of Al differ among soils, the main factor driving the concentration of Al in soil solution is pH. Soil acidity and associated Al toxicity inhibit the growth of roots, particularly fine ones, resulting in crops with stunted root systems. The stunted root systems have less capacity to take up water and nutrients from soil, often leading to lower yield potential.
Continued acidification of agricultural soils is due to unbalanced carbon and nitrogen cycles associated with product removal, the use of acidifying fertilisers and nitrate leaching. Efficient management of soil acidity is therefore critical to the ongoing profitability of cropping enterprises on soils that are prone to acidification, such as sandy soils in the wheatbelt with low buffering capacity. A series of experiments were conducted to provide new knowledge on how soil acidity can be efficiently managed in the wheatbelt of Western Australia. Some key findings of the work are summarised below.
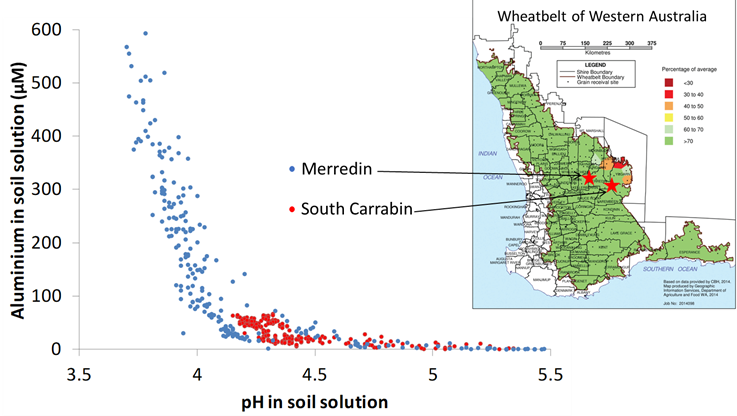
Figure 1: The pH and total dissolved aluminium concentration of soil solution extracted from two variously amended acidic, sandy soils from the eastern wheatbelt of south-western Australia. The soils were amended with various rates of a range of materials including carbonates, organics, biochar, gypsum and diatomaceous earth1.
Lime sources for neutralising soil acidity
Agricultural lime is the most effective material for correcting acidic soil pH and associated Al toxicity1. The effectiveness of agricultural lime in neutralising soil pH is primarily determined by its purity (calcium carbonate content), particle size distribution and incorporation into soil2,3. New technologies in the manufacture of very fine lime in liquid and prilled formulations overcome some obstacles associated with handling and application. However, there is little quantitative data on the effectiveness of these lime materials.
Glasshouse experiments with liquid and prilled formulations of very fine lime as well as a coastal limesand material reinforced that the neutralising value (calcium carbonate content) of the lime material was a primary factor determining its effectiveness in increasing soil pH4. Incorporation of lime through soil was also critical. Banding low rates of lime did not significantly influence soil pH or wheat growth and incorporating lime as intact prilled granules reduced its effectiveness due to limited distribution of lime in soil.
Lime particle size and soil moisture effects
The effectiveness of lime in neutralising acidic soil pH is dependent on the dissolution of the lime particles in soil. The rate of lime dissolution in soil is governed by various factors including the particle size (or fineness) of the lime material and the soil water content. The interaction of soil water content with the particle size distribution of lime was previously not known, and the effects on lime dissolution rates were not characterised. Experiments were conducted to quantify the effect of soil water content on the dissolution and effectiveness of i) very fine 0.05mm (range 0.045–0.063mm), ii) fine 0.20mm (range 0.180–0.212mm) and iii) coarse 0.50mm (range 0.420–0.500mm) lime particles. The 0.05mm particle size category represents the very fine limes that have been shown previously to be highly effective2,3 and are a characteristic component of some lime materials. The 0.20mm and 0.50mm particle size categories represent the major component and the coarse component, respectively, of many agricultural limes available in Western Australia5.
The 0.05mm lime particles dissolved rapidly within soil, resulting in large increases in soil pH (1.6 pH units) within seven days, regardless of the soil water content from 20 % to 80 % of water holding capacity (WHC) (Figure 2). Dissolution of the 0.5mm lime particles, on the other hand, was highly dependent on the soil water content. In very wet soil (80% of WHC), the coarse lime particles dissolved rapidly and extensively, and increased soil pH to a value approaching that achieved by the fine lime within 56 days. However, at a low soil water content (20% of WHC), the dissolution of the lime particles progressed slowly, as did the changes in soil pH. Dissolution of the 0.5mm lime progressed approximately 20-fold slower at the low (20% of WHC), compared to the high (80% of WHC) soil water content, with a half-life of 252 days (data not shown) versus 14 days. Like the 0.5mm particles, the dissolution of the 0.2mm lime particles was strongly influenced by the soil water content, being rapid and complete at the high water content (80% of WHC) and slow and incomplete at the low water content (20% of WHC). Although the dissolution of the 0.2mm and 0.5mm lime particles was statistically similar for most measurements, the 0.2mm lime induced a more rapid, significantly greater response in soil pH than the 0.5mm particles across the range of soil water contents.
Significant proportions of the 0.2mm and 0.5mm lime particles remained undissolved in soil at the low water content after 252 days (30% and 50% of the applied amount, respectively; data not shown). It is not clear whether these lime particles would continue to dissolve in soil (until completion), or whether the rate of dissolution will decrease with time. Predictions from iLime suggest that, for a sandy soil profile in the 325-400mm rainfall zone and a lime application rate of 4t/ha, a lime material composed entirely of particles <0.125mm will induce a rapid increase in soil pH in the 0–10 cm profile in year 1, with gradual declines thereafter. For lime materials composed entirely of particles in the 0.125–0.250mm or 0.250–0.500mm categories, iLime predicts soil pH in the 0–10cm profile will increase over a five-year period and be maintained at an elevated level for up to 20 years, suggesting a long-term benefit from ongoing dissolution of the lime particles.
Where subsoil horizons retain a greater water content than the surface layer, incorporation of lime into the subsoil may accelerate lime dissolution rates compared to lime residing in the surface layer of soil. In agricultural situations where lime materials containing very fine particles represent an economically viable option based on the bulk neutralising value and application constraints, they can effect rapid and sustained pH increases in soil with a low or high water content.
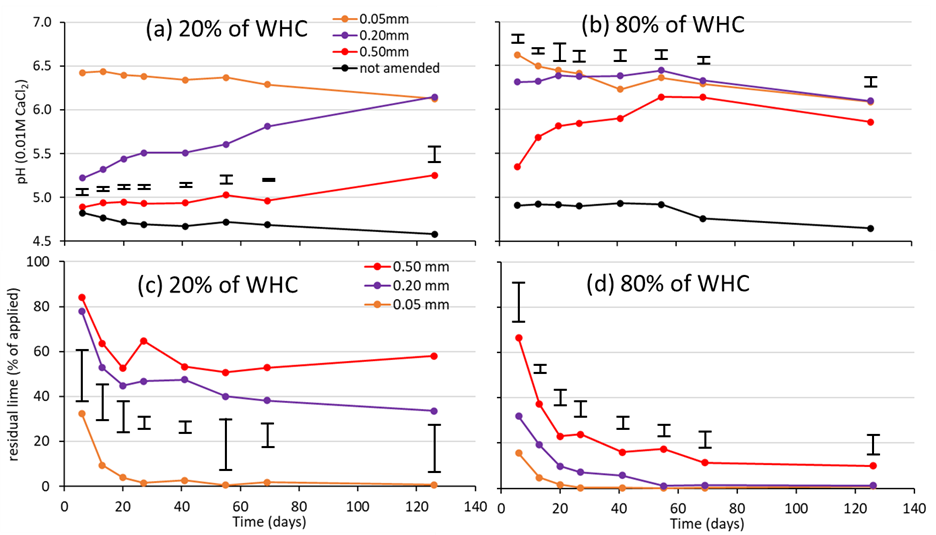
Figure 2: The pH of soil (in 0.01M CaCl2) incubated at (a) 20% and (b) 80% of water holding capacity (WHC) and undissolved, residual lime content at (c) 20% and (d) 80% of water holding capacity. The soil was collected from the 0–10cm horizon of an acidic, sandy profile at the Merredin Research Station. The water holding capacity of the soil was 25% (w/w). Lime particles (0.05mm, 0.20mm and 0.50mm size fractions) were applied at the rate of 1.0g CaCO3 equivalent/kg soil, which relates to approximately 1.4t/ha mixed to a depth of 10cm. The initial soil pHCaCl2 was 4.9. Soil was incubated at 15°C. The capped error bars are LSD (5%).
Improved root growth in limed soil leads to greater nutrient uptake
Soil acidity and Al toxicity can reduce the capacity of crops to take up nutrients and water from soil by inhibiting root growth. Likewise, ameliorating acidic, Al-toxic soils by liming can allow greater root proliferation and increase the capacity for nutrient and water uptake by crops6. An experiment was conducted to quantify the interaction between root proliferation and nutrient uptake in acidic, Al-toxic soil, or lime-amended soil. Wheat plants were grown in vertically split soil columns, with one half containing an acidic, Al-toxic subsoil and the other half containing the same subsoil amended with lime. Rubidium (Rb) was applied to either the acidic or lime-amended soil sections and its uptake into shoots was measured. Rubidium, which is considered a chemical analogue of potassium, is taken up by the same transport system as potassium and has been widely used as a tracer for potassium uptake from soil.
Root length within the limed soil sections was approximately eight-fold greater than in the acidic sections. Root length was correlated with the uptake of Rb, which was seven-fold greater from the limed soil sections than the acidic soil (Figure 3a). The Rb uptake per metre of root length was similar for the acidic or limed subsoil sections, regardless of the rate of Rb amendment or the location of the Rb placement (Figure 3b). The results consolidate previous evidence linking decreased nutrient uptake with stunted root systems in acidic, Al-toxic soil. Increased nutrient uptake from liming acidic soil is attributable to greater root length in the limed soil.
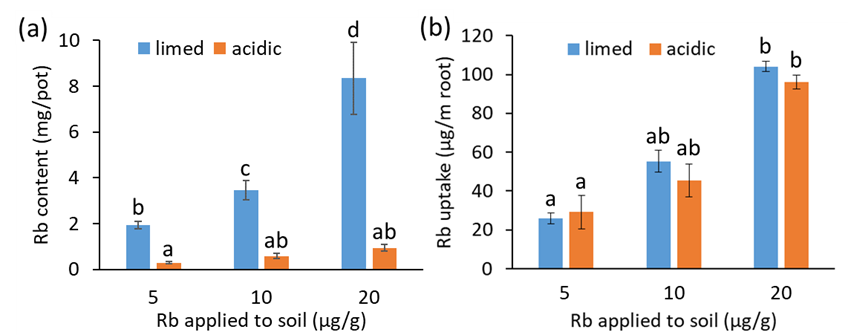
Figure 3: (a) Total rubidium (Rb) content in shoots of three-week-old wheat seedlings and (b) the uptake of Rb into shoots/m of root length within the Rb amended soil sections. Wheat plants were grown in vertically split soil columns, with an acidic (pHCaCl2 3.9) sandy subsoil in one half and the same subsoil amended with lime (pHCaCl2 6) in the other half. Rubidium chloride was applied to either the limed or unlimed sections of subsoil at 5, 10 or 20µg Rb/g soil and root length was measured within the soil sections to which Rb was applied. Capped error bars are ±SE, letters denote differences among means at p=0.05 (Tukeys HSD).
Proximity of crop rows to limed slots is the major factor influencing growth response
In soils with spatially heterogeneous distribution of soil with varied pH, the effects of soil acidity and Al toxicity on root growth are localised within the acidic sections of soil. Likewise, the effects of lime incorporation on the pH and Al concentration of soil solution are also localised within the amended soil sections1,4. Long-term field trials have shown that lime incorporated into acidic topsoil influenced soil pH within the tillage layer, but not within the subsoil below the tillage layer7,8, justifying strategic deep tillage to incorporate surface-applied lime into the acidic subsoil horizon.
Various soil tillage implements have been used to incorporate lime into acidic subsoil horizons, rapidly ameliorating acidity and Al toxicity. Lime slotting is one technique, whereby narrow seams of limed soil are incorporated into the subsoil, potentially to the depth of deep ripping. Experiments were conducted to assess the importance of factors such as the width, depth, and spacing of limed slots on the growth of wheat and canola in 50cm long, 30cm wide, 80cm deep acidic soil profiles in a glasshouse.
Experiments showed that the growth response of crop rows to lime slotting (40cm deep) was strongly influenced by their proximity to a limed slot (Figure 4a)9. The Rb tracer for root activity demonstrated that the proximity of a limed slot to a crop row influenced the timing at which the roots encountered it and exploited the resources within it (Figure 4b). For wheat rows positioned above or adjacent to a limed slot, their roots exploited it extensively within the first three weeks after sowing. A distance of 4, 9 or 11cm between the wheat row and the limed slot delayed the commencement of root activity within the limed slot by 2, 3 or 4 weeks, respectively. Consequently, wheat rows positioned above or adjacent to a limed slot were also able to access deeper subsoil horizons, beyond the depth of slotting, up to two weeks earlier than wheat rows positioned away from the limed slots.
Other work indicated that the width of limed slots was not a critical factor9, with similar wheat growth recorded in treatments with 4cm-wide or 8cm-wide limed slots. However, the depth of limed slots was important. Where limed slots were applied to a depth of 10cm, greater root proliferation within the limed soil did not extend below the depth of lime incorporation, and wheat growth was not significantly better than in a soil profile without lime amendment (data not shown).
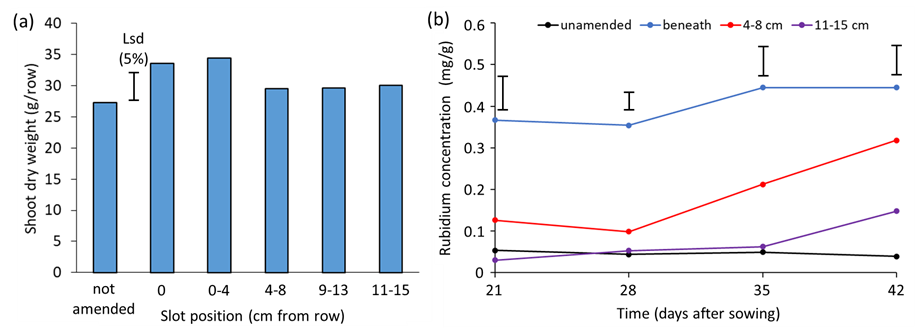
Figure 4: (a) Shoot dry weight at anthesis and (b) rubidium concentration in young leaves of wheat plants growing in acidic soil profiles in a glasshouse. The acidic soil profiles were amended with 4cm-wide, 40cm-deep slots of limed soil positioned at varying distances from the wheat rows, or not amended. The limed slots contained rubidium as a tracer for root activity within the amended soil sections (measured as the rubidium concentration in young leaves). Data for one wheat row per soil profile is shown. The capped error bars are LSD (5%).
Conclusions
Efficient management of soil pH requires knowledge of the processes that govern lime reactions in soil and subsequent crop responses. Lime incorporation into acidic soil increases soil pH, leading to greater proliferation of roots and greater uptake of nutrients and water from soil. However, lime effects are localised, and strategic tillage to incorporate lime into the subsoil is warranted.
Very fine (0.05mm) lime particles rapidly neutralise soil pH; however, the purchase cost and handling constraints associated with fine lime materials may be prohibitive. The dissolution of medium (0.2mm) and coarse (0.5mm) lime particles proceeds at a slower rate but can be accelerated significantly by incorporating the lime into soil layers with higher water content. Evidence from field trials suggests that the slower dissolution rate of larger lime particles can also be compensated for in the short term by higher rates of application.
If lime slotting is used to rapidly ameliorate acidic subsoil, yield responses will be maximised where wheat rows are positioned above the limed slots, which could be achieved if the spacing for tillage and seeding equipment are aligned.
The profitability of cropping enterprises on acidic soils will be maximised where efficient management of acidic soil pH is complemented with the use of crops and varieties tolerant to acid soils.
References
1 Damon P, Rengel Z, Azam G, Gazey C, Scanlan C, Malinowski D (2018a) Evaluating novel materials for ameliorating Al toxicity in acidic subsoils. In 2018 Grains Research Updates, Proceedings. GRDC Australia
2 Conyers, M. K., Scott, B. J., Fisher, R., & Lill, W. (1995). Predicting the field performance of twelve commercial liming materials from southern Australia. Fertilizer Research, 44(2), 151-161.
3 Whitten, M. (2001). Comparing size in lime. Journal of the Department of Agriculture, Western Australia, Series 4, 42(1), 10-14.
4 Damon P, Rengel Z, Azam G, Gazey C, Scanlan C, Malinowski D (2018b) Quantifying the effectiveness of prilled lime and liquid lime for neutralising soil acidity in the glasshouse. In 2018 Grains Research Updates, Proceedings. GRDC Australia
5 Gazey, C. (2013), Audit of WA agricultural lime quality 2013. Department of Agriculture and Food, Western Australia, Perth. Bulletin 4852
6 Scanlan, C. A., Brennan, R. F., D’Antuono, M. F., & Sarre, G. A. (2017). The interaction between soil pH and phosphorus for wheat yield and the impact of lime-induced changes to soil aluminium and potassium. Soil Research, 55(4), 341-353.
7 Reynolds, C., & Parker, W. (2018) Incorporating lime in order to ameliorate subsoil acidity faster. In 2018 Grains Research Updates, Proceedings. GRDC Australia
8 Azam, G., & Gazey, C. (2020). Slow movement of alkali from surface-applied lime warrants the introduction of strategic tillage for rapid amelioration of subsurface acidity in south-western Australia. Soil Research.
9 Damon P, Rengel Z, Azam G, Gazey C, Scanlan C, Malinowski D (2019) Lime slotting effects on wheat growth in an acidic soil profile. In 2019 Grains Research Updates, Proceedings. GRDC Australia
Acknowledgments
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC; the authors would like to thank them for their continued support. Bob Nixon (Kalannie), the Beck family (South Carrabin), DPIRD staff Alan Harrod, Gavin Sarre, Daron Malinowski and UWA staff Robert Creasy, Bill Piasini, Michael Smirk, Carolina Goes and Vivian Lis Pinero all provided significant support to the project.
Contact details
Paul Damon
UWA School of Agriculture and Environment
35 Stirling Hwy, CRAWLEY WA 6009
Ph: (+61)8 6488 2846
Email: paul.damon@uwa.edu.au
GRDC Project Code: DAW00252,
