Pulse disease research update
Pulse disease research update
Author: Joshua Fanning (Agriculture Victoria), James Manson (Southern Farming Systems), Blake Gontar (SARDI) and Jason Brand and Grant Hollaway (Agriculture Victoria) | Date: 24 Feb 2021
Take home messages
- Selecting a more resistant variety will reduce grain yield losses caused by disease.
- The faba bean, PBA Amberley will require fungicide application to prevent grain yield losses.
- Fungicide strategies incorporating newer fungicide actives are providing equal or better disease control than older actives in faba bean and chickpea.
- Applying fungicides to prevent ascochyta blight in chickpea when grain yields are above 0.5t/ha resulted in an economic advantage in field experiments.
- Inter-row sowing into standing cereal stubble, compared to slashed stubble, is almost as effective at reducing chickpea grain yield losses due to ascochyta blight, as choosing a moderately susceptible variety compared to a susceptible variety.
- Numerous pathogens have been detected in pulse roots, and many Fusarium spp. and Phoma/Didymella spp. have been shown to cause root disease.
Background
Management of pulse diseases is essential to on-farm profitability, and this was especially the case during 2020. Consistent winter rainfall and foggy conditions provided conducive conditions for disease development in Victoria. In field experiments, 35% loss in grain yield was measured in faba bean at Lake Bolac (high rainfall) and 92% in the susceptible chickpea variety PBA Striker at Curyo (low rainfall), highlighting the importance of controlling pulse diseases in a range of environments.
The following report will provide an update on experiments investigating;
- Effect of faba bean plant densities on disease severity,
- Disease management options in chickpea and faba bean varieties utilising varietal resistance and fungicides,
- The interaction of stubble height on chickpea ascochyta blight management, and
- Soil-borne diseases in pulse crops.
Faba bean disease management
Sowing rate
Chocolate spot in faba bean is a large threat to yield, particularly in high rainfall areas. Chocolate spot disease development can occur at most growing season temperatures, but disease development is quickest when canopy humidity is high (greater than 70%) and temperatures are warm (15-25°C). These environmental conditions can differ between crops, depending on the prevailing weather but also the canopy density.
Field experiments at Lake Bolac and Lake Linlithgow showed that chocolate spot severity increased in faba bean plots as target plant densities increased from 5 to 45 plants per square metre, with similar results observed at both sites (Figure 1). The variety PBA BendocA (rated moderately susceptible (MS)) had greater disease severity in all plant densities, compared to PBA Amberley (provisionally rated moderately resistant (MR)). Additionally, PBA Amberley had over 60% disease severity in high density (45 plants/m2) plots, equivalent to PBA Bendoc at a low density of 5-15 plant/m2. This highlights that the ideal conditions for disease will still put pressure on the moderate resistance in PBA Amberley and result in grain yield losses. The chocolate spot resistance rating of faba bean is reviewed annually, so it is important to always check for up to date disease ratings.
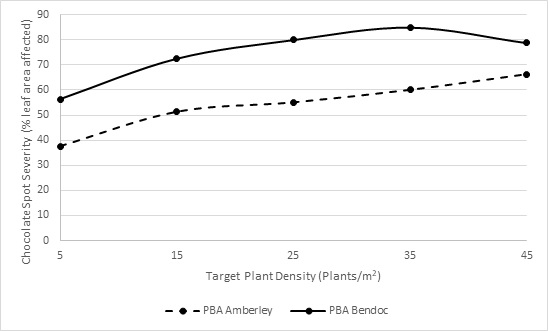
Figure 1. Increasing plant density resulted in increasing chocolate spot severity in unsprayed plots atLake Bolac in a field trial planted 9 April 2020.
Varietal resistance and fungicide strategy
With the recent release of PBA Amberley (provisionally rated MR to chocolate spot) and the newer fungicide actives, it was important to review fungicide strategies against varietal resistance to determine if the number of applications required to prevent grain yield loss can be reduced. At five locations across Victoria (Dookie, Nhill, Gymbowen, Lake Linlithgow and Lake Bolac) experiments were conducted to compare varietal resistance and fungicide strategies. Only the Lake Bolac and Gymbowen results are presented in this paper. Newer chemistries including, tebuconazole + azoxystrobin (Veritas®), bixafen + prothioconazole (Aviator® Xpro®) and fludioxonil + pydiflumetofen (Miravis Star® - registration submission awaiting approval from the APVMA) were compared against older chemistries including carbendazim or procymidone (Table 1). The aim was to compare the newer chemistries applied at early flowering with the older chemistries at canopy closure. All treatments received a 4-node tebuconazole application to prevent cercospora leaf rot. Treatments were applied at early flowering as these newer chemistries were expected to have longer efficacy and this timing is the latest some of these products can be applied to comply with label directions. The Lake Bolac and Gymbowen experiments were sown 19 April and 23 April 2020, respectively.
At Lake Bolac, additional applications of carbendazim and procymidone were applied in addition to the fungicide treatments, as conducive disease conditions continued throughout the season and this site needed additional fungicide controls. In other experiments on these sites, it was difficult to control chocolate spot in susceptible varieties, even with fungicides. This highlights the need for proactive disease management as disease epidemics can develop rapidly.
Table 1. Fungicide treatments and timings in faba bean experiments conducted at Lake Bolac and Gymbowen during 2020.
| TreatmentA | Rate (gai/ha) | Timing |
|---|---|---|
| Untreated (no fungicides) | ||
| Carbendazim | 250 | Canopy closure |
| Procymidone | 250 | Canopy closureB |
| Tebuconazole + Azoxystrobin | 200 120 | Early flowering |
Bixafen + Prothioconazole | 45 90 | Early flowering |
Fludioxonil + PydiflumetofenC | _C | Early flowering |
| Full ControlD |
AThese fungicides are additional to all treatments receiving a tebuconazole application at the 4 to 6 node growth stage. At Lake Bolac there was significant disease pressure later in the season. Therefore, an additional two carbendazim (250 gai/ha) and an extra one procymidone (240 gai/ha, two extra procymidone applications on the carbendazim treatment) were applied alternately every 2-4 weeks to control the chocolate spot; BAt Lake Bolac the first procymidone was applied at flowering; CThis is a new product to Australia called Miravis Star® which is currently going through the registration and approval process; DThe full control treatment is a rotation of fungicides applied to ensure minimal to no disease as a control in the experiment.
Disease epidemics varied between locations with no disease observed at Nhill and Dookie. Gymbowen, Lake Bolac and Lake Linlithgow all had chocolate spot, and a low level of cercospora leaf spot. All three sites with chocolate spot demonstrated similar results, so Lake Bolac is highlighted where disease was moderate (Table 2). PBA Bendoc had consistently more severe chocolate spot compared to the other varieties, with disease symptoms observed and progressing under the ‘Full Control’ fungicide treatment. In comparison, PBA Samira and PBA Amberley had the least disease severity. The fungicide treatment containing Fludioxonil + Pydiflumetofen resulted in disease severity similar to the ‘Full control’. These results highlight the requirement for fungicides to be applied to PBA Amberley to prevent severe disease.
Faba bean grain yield recorded at Lake Bolac and Gymbowen indicated greater fungicide efficacy in the newer chemistries, with Fludioxonil + Pydiflumetofen, providing higher yield gains compared to the other fungicide strategies. Economic benefit of applying the new chemistries, was similar to the older strategy involving carbendazim (Table 3 and 4).
Table 2. Chocolate spot severity in four varieties with different fungicide strategies applied at Lake Bolac, assessed on 20 October 2020.
TreatmentA | Chocolate Spot Severity (%) | ||||
|---|---|---|---|---|---|
Fiesta | PBA Bendoc | PBA Samira | PBA Amberley | Mean | |
Untreated | 64 | 65 | 54 | 56 | 60 a |
Carbendazim | 35 | 41 | 25 | 26 | 32 c |
Procymidone | 46 | 60 | 41 | 33 | 45 b |
Tebuconazole + Azoxystrobin | 48 | 61 | 39 | 28 | 44 b |
Bixafen + Prothioconazole | 43 | 61 | 36 | 24 | 41 b |
Fludioxonil + Pydiflumetofen | 16 | 23 | 13 | 6 | 14 d |
Full control | 11 | 28 | 7 | 3 | 12 d |
Mean | 37 b | 48 a | 31 c | 25 c | |
P | LSD | ||||
Variety | <0.001 | 5.8 | |||
Treatment | <0.001 | 7.7 | |||
Variety x treatment interaction | 0.811 | ns | |||
AThese fungicides treatments are in addition to the tebuconazole application at the 4-6 node stage described in Table 1.
Table 3. Grain yield of four varieties, with seven different fungicide strategies applied at Lake Bolac during 2020. Percentage yield increase relative to untreated and economic advantage ($/ha) of each fungicide treatment is also presented.
TreatmentA | Grain Yield (t/ha) | Yield Increase | Mean Economic AdvantageC | ||||
|---|---|---|---|---|---|---|---|
Fiesta | PBA Bendoc | PBA Samira | PBA Amberley | MeanB | |||
Untreated | 3.03 | 3.28 | 3.73 | 3.66 | 3.42 a | 0% | |
Carbendazim | 3.96 | 4.25 | 4.90 | 4.25 | 4.34 b | 27% | $258 |
Procymidone | 4.23 | 4.30 | 4.90 | 4.9 | 4.58 b | 34% | $371 |
Tebuconazole + Azoxystrobin | 3.91 | 4.30 | 4.80 | 4.4 | 4.35 b | 27% | $266 |
Bixafen + Prothioconazole | 4.05 | 4.47 | 4.67 | 4.58 | 4.44 b | 30% | $294 |
Fludioxonil + Pydiflumetofen | 4.98 | 5.45 | 4.97 | 5 | 5.10 c | 49% | |
Full control | 4.78 | 5.74 | 5.17 | 5.4 | 5.27 c | 54% | |
Mean | 4.14 a | 4.54 b | 4.73 b | 4.60 b | |||
P | LSD | ||||||
Variety | <0.001 | 0.264 | |||||
Treatment | <0.001 | 0.350 | |||||
Variety x treatment interaction | 0.49 | ns | |||||
AThese fungicides treatments are in addition to the tebuconazole application at the 4-6 node stage described in Table 1; BDifferent letters indicate pairwise significance (P<0.05); CEconomic advantage was calculated as the grain yield gains minus the cost of the fungicide treatments. Chemical prices were an average of three chemical resellers prices provided, grain price was assumed to be $400/ton, and an application cost of $10/ha.
Table 4. Grain yield of four varieties, with seven different fungicide strategies applied at Gymbowen during 2020. Percentage yield increase relative to untreated and economic advantage ($/ha) of each fungicide treatment is also presented.
TreatmentA | Grain Yield (t/ha) | Yield Increase | Mean Economic AdvantageC | ||||
|---|---|---|---|---|---|---|---|
Farah | PBA Bendoc | PBA Samira | PBA Amberley | MeanB | |||
Untreated | 6.31 | 6.13 | 6.38 | 5.93 | 6.19 a | 0% | |
Carbendazim | 6.80 | 6.72 | 7.14 | 6.98 | 6.91 bc | 12% | $258 |
Procymidone | 6.59 | 6.72 | 6.98 | 6.67 | 6.74 b | 9% | $182 |
Tebuconazole + Azoxystrobin | 7.00 | 6.61 | 7.04 | 7.22 | 6.97 bc | 13% | $260 |
Bixafen + Prothioconazole | 6.79 | 6.80 | 7.48 | 6.95 | 7.01 bc | 13% | $269 |
Fludioxonil + Pydiflumetofen | 7.08 | 6.73 | 7.20 | 7.22 | 7.06 c | 14% | |
Full control | 7.21 | 7.25 | 6.74 | 7.39 | 7.15 c | 16% | |
Mean | 6.83 | 6.71 | 6.99 | 6.91 | |||
P | LSD | ||||||
Variety | 0.072 | ns | |||||
Treatment | <0.001 | 0.290 | |||||
Variety x treatment interaction | 0.295 | ns | |||||
AThese fungicides treatments are in addition to the tebuconazole application at the 4-6 node stage described in Table 1; BDifferent letters indicate pairwise significance (P<0.05); CEconomic advantage was calculated as the grain yield gains minus the cost of the fungicide treatments. Chemical prices were an average of three chemical resellers prices provided, grain price was assumed to be $400/ton, and an application cost of $10/ha.
Disease management of ascochyta blight in chickpea
Varietal resistance and fungicide strategy
New fungicide chemistries for use in pulses may have curative effects and could potentially reduce fungicide use in chickpea to control ascochyta blight. Experiments were established to determine the newer chemistries’ efficacy applied after the signs of ascochyta blight infection compared with the traditional control method of preventative fungicide applications. These newer fungicides were also compared to older chemistries applied before infection (Table 5). Experiments were conducted from 2018 to 2020 at Curyo and Horsham.
Disease epidemics were observed at Curyo and Horsham in each year, resulting in significant grain yield losses (Table 6 and 7). The grain yield results highlight the importance of selecting a less susceptible variety such as Genesis 090 compared to the susceptible variety PBA StrikerA and the importance of using fungicides to prevent/control disease.
The calculated figures for the economic advantage show that in most years the application of any fungicide is more profitable than not applying fungicides (Table 8). However, under low yield potential conditions associated with low rainfall, such as at Curyo in 2018 (<0.5t/ha), fungicide application treatments were not economically beneficial. In comparison, at the Horsham site in 2018, grain yields were slightly greater than 0.5t/ha, and the application of fungicides resulted in an economic benefit.
These results also highlight the potential use of a post-infection fungicide application in lower rainfall environments such as the Victorian Mallee. During 2018-2020, the post-infection fungicide applications were profitable at both sites. In paddock situations, particularly in lower rainfall environments, where disease pressure may be lower, these post infection fungicide applications may provide a lower input system for growers. However, growers must be ready to apply fungicides in a timely manner. Additionally, post-infection fungicide applications are a higher risk strategy as disease severity can increase quickly in conducive conditions, and therefore, care must be taken if following this fungicide application strategy.
Table 5. Fungicide treatments and the number of applications applied for each treatment to assess control of ascochyta blight (AB) in chickpea at Curyo and Horsham during 2018-2020. All treatments had a Thiram (0.72 gai / kg seed) + Thiabendazole (0.4 gai / kg seed) seed treatment applied.
In Season Fungicide | Rate (g ai/ha) | TimingB | Number of Sprays | |||||
|---|---|---|---|---|---|---|---|---|
Curyo | Horsham | |||||||
18D | 19 | 20 D | 18 D | 19 | 20 | |||
ACaptanTM | 1000 | Strategically | 2+1 | 2 | 2+1 | 2+1 | 2 | 2 |
Chlorothalonil | 1080 | Strategically | 3+1 | 2 | 2+1 | 2+1 | 2 | 2 |
Tebuconazole Azoxystrobin | 200 120 | Strategically | 2+1 | 2 | 2+1 | 2+1 | 2 | 2 |
Bixafen Prothioconazole | 45 90 | Strategically | 2+1 | 2 | 2+1 | 2+1 | 2 | 2 |
Tebuconazole + Azoxystrobin | 200 120 | Post Infection | 1+1 | 1 | 2+1 | 1+1 | 1 | 1 |
Bixafen Prothioconazole | 45 90 | Post Infection | 1+1 | 1 | 2+1 | 1+1 | 1 | 1 |
Full ControlC | ||||||||
ACaptan is registered for control of Ascochyta blight in chickpeas at this rate under permit PER81406; BStrategic sprays were applied before rainfall events, at key growth stages (4th node and late vegetative / early flowering stage), to maximise foliage protection. Post infection sprays were applied when the first AB lesions were observed and at flowering. Trials were inspected at least weekly. CThe full control treatment is a rotation of fungicides applied to ensure minimal to no disease as a control in the experiment. In addition to the fungicides listed an additional podding Chlorothalonil at 1080 gai/ha was applied to protect seed quality.
Table 6. Yield response of two chickpea varieties to 10 different fungicide application strategiesat Curyo during 2018 to 2020. Where there was a significant difference (P<0.05) the percentage grain yield gain was calculated. Different letters indicate pairwise significance (P<0.05). All treatments (except untreated) had a Thiram + Thiabendazole seed treatment applied.
Treatment | Timing | 2018 | 2019 | 2020 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Grain Yield (t/ha) | Yield Gain | Grain Yield (t/ha) | Yield Gain | Grain Yield (t/ha) | Yield Gain | |||||||||
Genesis 090 | PBA Striker | Mean | Mean | Genesis 090 | PBA Striker | Mean | Mean | Genesis 090 | PBA Striker | Mean | Genesis 090 | PBA Striker | ||
Untreated | 0.37 | 0.37 | 0.37 a | 0% | 1.35 | 0.56 | 0.95 a | 0% | 1.02 cd | 0.22 a | 0.62 | 0% | 0% | |
Captan | Strategically | 0.49 | 0.48 | 0.49 c | 39% | 1.36 | 0.88 | 1.12 ab | 39% | 1.53 efg | 0.51 ab | 1.02 | 138% | 98% |
Chlorothalonil | 0.51 | 0.46 | 0.49 c | 39% | 1.57 | 1.16 | 1.37 bcd | 39% | 2.11 i | 1.41 def | 1.76 | 553% | 159% | |
Tebuconazole + Azoxystrobin | 0.56 | 0.50 | 0.53 c | 53% | 1.63 | 1.07 | 1.35 bcd | 53% | 1.8 fghi | 0.94 bcd | 1.37 | 335% | 248% | |
Bixafen + Prothioconazole | 0.46 | 0.43 | 0.45 abc | 26% | 1.65 | 1.30 | 1.48 cd | 26% | 2.03 hi | 1.28 de | 1.66 | 494% | 293% | |
Tebuconazole + Azoxystrobin | Post-Infection | 0.49 | 0.44 | 0.47 bc | 32% | 1.56 | 0.94 | 1.25 bc | 32% | 1.98 ghi | 1.29 de | 1.63 | 497% | 126% |
Bixafen + Prothioconazole | 0.51 | 0.45 | 0.48 bc | 36% | 1.76 | 1.20 | 1.48 cd | 36% | 1.78 fghi | 1.24 de | 1.51 | 476% | 174% | |
Full Control | 0.47 | 0.48 | 0.47 bc | 35% | 1.94 | 1.87 | 1.9 e | 35% | 2.65 j | 2.86 j | 2.75 | 159% | 1222% | |
Mean | 0.48 a | 0.45 b | 1.63 a | 1.11 b | 1.86 | 1.15 | ||||||||
P | LSD | P | LSD | P | LSD | |||||||||
Variety | 0.062 | 0.036 | <0.001 | 0.063 | <0.001 | 0.149 | ||||||||
Treatment | 0.010 | 0.080 | <0.001 | 0.140 | <0.001 | 0.334 | ||||||||
Variety x treatment interaction | 0.991 | 0.114 | 0.412 | ns | 0.030 | 0.472 | ||||||||
Table 7. Yield response of two chickpea varieties to 10 different fungicide application strategies at Horsham during 2018 to 2020. Where there was a significant difference (P<0.05) the percentage grain yield gain was calculated. Different letters indicate pairwise significance (P<0.05). All treatments (except untreated) had a Thiram + Thiabendazole seed treatment applied.
Treatment | Timing | 2018 | 2019 | 2020 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Grain Yield (t/ha) | Yield Gain | Grain Yield (t/ha) | Yield Gain | Grain Yield (t/ha) | Yield Gain | |||||||||
Genesis 090 | PBA Striker | Mean | Mean | Genesis 090 | PBA Striker | Mean | Mean | Genesis 090 | PBA Striker | Mean | Genesis 090 | PBA Striker | ||
Untreated | 0.42 | 0.18 | 0.3 a | 0% | 0.79 | 0.57 | 0.68 a | 0% | 1.58 de | 0.44 a | 1.01 | 0% | 0% | |
Captan | Strategically | 0.53 | 0.41 | 0.47 b | 58% | 1.02 | 0.69 | 0.85 ab | 26% | 1.82 efgh | 0.86 b | 1.34 | 16% | 98% |
Chlorothalonil | 0.50 | 0.49 | 0.49 bc | 66% | 1.81 | 1.24 | 1.53 cd | 126% | 2.26 ij | 1.13 bc | 1.69 | 43% | 159% | |
Tebuconazole + Azoxystrobin | 0.61 | 0.39 | 0.5 bc | 69% | 1.45 | 1.01 | 1.23 bcd | 82% | 2.11 fghi | 1.52 cde | 1.81 | 34% | 248% | |
Bixafen + Prothioconazole | 0.58 | 0.52 | 0.55 bcd | 84% | 2.36 | 2.36 | 2.36 e | 249% | 2.30 ij | 1.71 ef | 2.01 | 46% | 293% | |
Tebuconazole + Azoxystrobin | Post-Infection | 0.60 | 0.50 | 0.55 bcd | 85% | 1.25 | 0.76 | 1.00 abc | 49% | 1.73 efg | 0.98 b | 1.36 | 10% | 126% |
Bixafen + Prothioconazole | 0.60 | 0.51 | 0.56 bcd | 87% | 1.79 | 1.33 | 1.56 d | 131% | 1.69 e | 1.19 bcd | 1.44 | 7% | 174% | |
Full Control | 0.65 | 0.60 | 0.62 d | 108% | 2.06 | 2.19 | 2.12 e | 214% | 2.56 j | 2.33 ij | 2.45 | 62% | 436% | |
Mean | 0.57 a | 0.45 b | 1.60 a | 1.22 b | 2.04 | 1.27 | ||||||||
P | LSD | P | LSD | P | LSD | |||||||||
Variety | <0.001 | 0.093 | 0.002 | 0.234 | <0.001 | 0.129 | ||||||||
Treatment | <0.001 | 0.042 | <0.001 | 0.523 | <0.001 | 0.289 | ||||||||
Variety x treatment interaction | 0.303 | ns | 0.772 | ns | 0.049 | 0.409 | ||||||||
Table 8. Economic advantage ($/ha) of foliar fungicide applications to control chickpea Ascochyta blight at Curyo and Horsham during 2018 to 2020. Economic advantage was calculated as the grain yield gains minus the cost of the fungicide treatments. Chemical prices were an average of three chemical resellers prices provided, grain price was assumed to be $600/t, and an application cost of $10/ha.
Treatment | Timing | Curyo | Horsham | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
2018 | 2019 | 2020 | 2018 | 2019 | 2020 | |||||||
Mean | Mean | Genesis 090 | PBA Striker | Mean | Mean | Genesis 090 | PBA Striker | |||||
Captan | Strategically | $8 | $40 | $205 | $81 | $6 | $45 | $85 | $195 | |||
Chlorothalonil | -$36 | $178 | $548 | $611 | $12 | $439 | $337 | $343 | ||||
Tebuconazole + Azoxystrobin | $24 | $168 | $357 | $329 | $18 | $263 | $251 | $578 | ||||
Bixafen + Prothioconazole | -$39 | $229 | $480 | $520 | $29 | $924 | $349 | $678 | ||||
Tebuconazole + Azoxystrobin | Post-Infection | $23 | $143 | $468 | $539 | $81 | $162 | $56 | $294 | |||
Bixafen + Prothioconazole | $22 | $273 | $332 | $496 | $78 | $488 | $24 | $410 | ||||
Standing stubble can reduce ascochyta blight
In the last several years it has been observed that when planting chickpea into cereal stubble rows, ascochyta blight spread was confined to the row. Adjacent rows often remained disease free.
The effect of inter-row sowing chickpea into standing (30cm tall) versus slashed cereal stubble, with two chickpea varieties (PBA Striker (S) and Genesis 090 (MS)), and a complete disease control (no disease/full fungicide) or untreated (diseased/no fungicide) plots was investigated. To inoculate the disease plots, six ascochyta infested stubble pieces (10cm length) were pinned in the geometric centre of plots. The ascochyta blight severity and grain yield were measured.
Inter-row sowing into standing cereal stubble resulted in 31% yield loss due to ascochyta blight compared to 55% in slashed stubble (Table 9). In this experiment, the magnitude of the yield increase due to standing stubble was almost as much as changing from a susceptible variety to a moderately susceptible variety (Table 10).
These results highlight the importance of not only selecting more resistant varieties but combining this with good agronomy practices.
Table 9. Grain yield of chickpea inter-row sown into standing versus slashed cereal stubble with and without disease at Dooen during 2020. There was a significant interaction (P=0.001) between stubble and fungicide. Different letters indicate a significant difference in a pairwise analysis between fungicide and stubble.
Fungicide | Stubble | |
|---|---|---|
Slashed | Standing | |
Disease Free | 2.10 c | 2.00 c |
Diseased | 0.96 a | 1.38 b |
Yield Loss (t/ha) | 1.15 | 0.62 |
Yield Loss (%) | 55% | 31% |
Table 10. Grain yield of two chickpea varieties with and without disease at Dooen during 2020. There was a significant interaction (P<0.001) between variety and fungicide. Different letters indicate a significant difference in a pairwise analysis between fungicide and stubble.
Fungicide | Variety | |
|---|---|---|
Genesis090 | PBA Striker | |
Disease Free | 2.16 d | 1.95 c |
Diseased | 1.56 b | 0.78 a |
Yield Loss (t/ha) | 0.61 | 1.16 |
Yield Loss (%) | 28% | 60% |
Soil-borne disease
Growers and agronomists have reported patches of poor performing crops and, in some situations, crop failures. Preliminary investigations into these poor performing areas identified that soil-borne disease may be the cause. In response to these concerns, a national soil-borne disease project investigated the presence of soil-borne pathogens (pathogens cause disease) on pulse crops.
Detecting pathogens in pulse roots
Throughout Australia, root samples of poor performing pulse crops were sent to local pathologists for visual assessment and then to SARDI for molecular identification of pathogens present. In 2020, 533 samples were processed, root health was scored and photographed, and molecular techniques were used to determine the pathogens present in pulse crops.
The most common pathogens detected using qPCR were Pythium spp., Pratylenchus spp. (root lesion nematodes), Rhizoctonia solani AG8, and Phoma pinodella. Pythium and Pratylenchus spp. are known to have broad host ranges. R. solani AG8 prefers cereals but will infect a broad range of plant types. Phoma pinodella along with Didymella pinodes causes blackspot of field pea, but it has a much broader host range. These pathogens are very often present together in the one root system (‘disease complex’).
There were also infrequent detections of Aphanomyces and Phytophthora genera. These genera have been reported to cause severe and widespread yield losses in pulses in Europe and North America. During 2020, Aphanomyces euteiches was detected in six faba/broad bean samples from South Australia (SA) and New South Wales (NSW) and one lentil sample from Victoria; the collecting agronomists reported significant yield loss in many of these paddocks.
Phytophthora medicaginis, a known problem in northern NSW, was detected in 26 (25 chickpea, 1 faba bean) samples from northern NSW; P. megasperma was detected in 33 samples (multiple crop types) across Australia, and P. drechsleri (tentative identification), was detected in 14 samples, mostly lupins from Western Australia (WA); this species was also detected in SA, Victoria and southern NSW. SARDI is currently undertaking work to confirm the identity of this species.
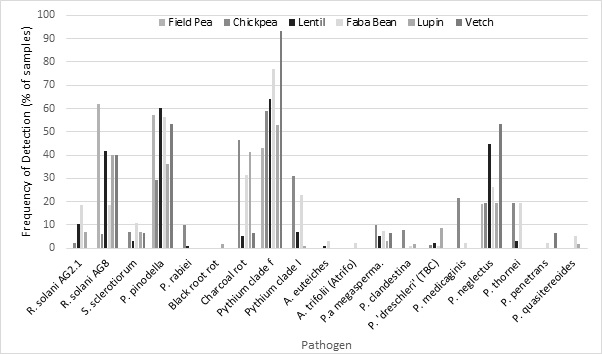
Figure 2. Frequency of detection over threshold levels of pathogens using molecular tools in pulse samples received nationally during 2020.
Confirming the cause of disease
To confirm the role of specific organisms in causing root diseases in pulses, the pathogens were isolated from the diseased pulse samples and then inoculated onto soil, in which various pulses were grown. Pathogens which re-infect the seedlings are termed pathogenic. This study aimed to identify and confirm pathogenicity of isolates that are likely to be the main causes of symptoms observed in growers’ paddocks.
Fusarium avenaceum isolates were highly pathogenic on all crops tested, with just one strain appearing non-pathogenic (Table 11). Fusarium oxysporum and F. redolens isolates’ pathogenicity varied between crops, but all isolates of both species were highly pathogenic on chickpea. Fusarium tricinctum isolates were highly pathogenic on chickpea and moderately pathogenic on faba bean, lentil and lupin. Phoma. pinodella isolates were highly pathogenic on chickpea, field pea and lentil, but less so on faba bean and lupin (Table 12).
Further work will investigate yield losses and control options to ensure growers have management options to reduce grain yield losses due to soil-borne diseases.
Table 11. Frequency of detection of Fusarium spp. in 2019 survey samples and pathogenicity (ability of the pathogen to infect plant) of representative isolates collected from 2018-2019 crop samples. Pathogenicity on each crop is indicated as “-“ non-pathogenic, “+” weakly pathogenic, “++” moderately pathogenic or “+++” highly pathogenic.
Species | Isolates Collected | Isolates Tested | Chickpea | Field pea | Faba bean | Lupin | Lentil |
|---|---|---|---|---|---|---|---|
F. acuminatum | 12 | 5 | 56% | 77% | 84% | 71% | 89% |
-/+ | -/+ | -/+ | -/+ | -/+ | |||
F. avenaceum | 6 | 5 | 6% | 31% | 63% | 29% | 27% |
-/+++ | -/+++ | +/+++ | -/+++ | -/+++ | |||
F. culmorum | 1 | 1 | 6% | 8% | 21% | 7% | 6% |
++/+++ | +/+++ | +/+++ | +/+++ | -/+ | |||
F. equiseti | 4 | 4 | 48% | 69% | 72% | 55% | 49% |
-/++ | -/+ | -/++ | -/+ | - | |||
F. oxysporum | 17 | 14 | 91% | 100% | 91% | 80% | 73% |
++/+++ | -/+ | -/+ | -/++ | -/++ | |||
F. redolens | 2 | 2 | 31% | 0% | 16% | 9% | 29% |
++/+++ | -/+ | -/++ | -/++ | -/+ | |||
F. solani | 4 | 4 | 70% | 0% | 19% | 36% | 13% |
-/+ | -/+ | -/+ | -/+ | -/+ | |||
F. tricinctum | 4 | 4 | 5% | 31% | 37% | 30% | 2% |
+++ | -/+ | -/++ | +/++ | +/++ |
Table 12. Frequency of detection of Phoma spp. in 2019 survey samples and pathogenicity (ability of the pathogen to infect plant) of P. pinodella isolates collected from samples 2018-2019 toward common pulse crops in a controlled environment assay. Pathogenicity is indicated as either non-pathogenic “-“, weakly pathogenic “+”, moderately pathogenic “++” or highly pathogenic “+++”.
Species | Unique Isolates | Isolates Tested | Chickpea | Field pea | Faba bean | Lupin | Lentil |
|---|---|---|---|---|---|---|---|
Phoma/Didymella spp. | 20 | 16 | 53.13% | 100.00% | 90.70% | 67.86% | 75.00% |
++/+++ | ++/+++ | +/++ | -/++ | ++/+++ |
Conclusion
Without a disease management plan that incorporates varietal resistance, paddock rotations, good agronomy practices and fungicides, grain yield losses of greater than 90% can be experienced. The results from these projects show the importance of varietal resistance in both chickpea and faba bean to minimise grain yield losses. The importance of fungicides, both type and timing of application play a critical role in disease control. However, the importance of good agronomy cannot be understated with ensuring that disease management plans incorporate planting densities and orientation of stubble (standing versus slashed). Inter-row sowing chickpea into standing cereal stubble was shown to be almost as effective as changing resistance classification of the variety from S to MS as compared to sowing into slashed stubble.
Investigations into soil-borne diseases in pulses are still continuing, with both Fusarium spp. and Phoma/Didymella spp. identified as pathogens of significance. Following these investigations, control methods will be investigated.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support. This work is supported by both the GRDC and the Victorian Government (Agriculture Victoria) through the GRDC projects; DJP1097-001RTX, DAV00150, DJP1907-002RMX, DJP1907-004RTX.
Appreciation is given to the Agriculture Victoria Southern Pulse Agronomy, Field Crops Pathology, Southern Farming Systems and SARDI research teams and the growers and agronomists who have assisted with these experiments.
Useful resources
Crop Protection Products including Minor Use Permits, can be viewed at the Australian Pesticides and Veterinary Medicines Authority (APVMA) website
Contact details
Joshua Fanning
110 Natimuk Road, Horsham Vic 3400
03 5344 3335
Joshua.fanning@agriculture.vic.gov.au
@FanningJosh_
GRDC Project Code: DJP1097-001RTX, DAV1706-003RMX, DJP1907-002RMX, DJP1907-004RTX,
