The potential of deep ripping heavy, sodic soil with nutrient burial
The potential of deep ripping heavy, sodic soil with nutrient burial
Author: Wayne Parker (Department of Primary Industries and Regional Development) | Date: 11 Feb 2021
Key messages
- Deep ripping and deep nutrient placement in sodic heavy soil can increase the number of crop roots deeper in the profile.
- The Howard Paraplow®, slant tine deep ripper, reduced subsoil clods brought to the surface as occurs with straight shank deep rippers.
- Cloddiness from deep ripping heavy soil needs to be reduced to allow plant emergence to be equivalent to non-ripped soil.
Aims
To improve root exploration of a sodic clay soil with the addition of deep placed nutrients via deep ripping.
Introduction
There is a recognised challenge in transferring the deep ripping technique from sand to sodic clay soil in Western Australia and achieving a consistent increase in yield. This is due to behaviour of the soil when ripping and the poor physical and chemical properties of the clay at depth (Moore 2004). Excessive cloddiness after the fracturing process interferes with seed placement at sowing. Elevated levels of boron, magnesium and sodium result in soil physical and chemical limitations to crop growth. Elevated sodium levels in a clay soil negatively influence the structure of the soil to create conditions that are hostile to root growth (Hamilton et al 2005), while other salts and boron are often at levels that are toxic to crops.
Deep ripping of sodic soil with higher clay content is not a recommended practice as the soil slumps upon wetting up, clay is dispersed filling pores and settling in a massive, plated structure as it dries. These structures are high in strength, which has negative implications for root growth. The clods remaining after deep ripping sodic soil also provide challenges for emerging seedlings with low establishment rates common.
Sodic soil is characterised by high strength, lack of structure and high sodium concentration. The intent of deep ripping is to reduce high soil strength, improve structure and provide root pathways into the soil. Gypsum is a source of calcium used to improve structure of sodic loam and clay soil through displacement of sodium from clay surfaces. Plant roots and organic matter are biological methods that help provide structure and new root pathways through hostile zones in the soil. Long phases of lucerne pasture help growers to crop sodic soil as the remnant lucerne root channels can assist root growth into the subsoil of following crops (Nuttall et al 2008). There are positive interactions between ripping and deep placement of organic amendments that improve structure and yield potential of sodic clay subsoil (Tavakkoli et al 2019).
This paper explores the use of deep-placed nutrients to increase root penetration within sodic subsoil using a slant tine deep ripper. Nitrogen and gypsum were the two nutrients selected; nitrogen as a root growth stimulant and gypsum to provide channels of structured soil through sodic layers. The slant tine ripper was used to minimise surface cloddiness.
In addition to reporting on two trials conducted in 2020 this paper draws on deep ripping trials conducted in the GRDC-supported project DAW00243 using topsoil slotting plates.
Method
The ripping at Mingenew and Canna was implemented using a modified Howard Paraplow® slant tine deep ripper. The three-tine ripper was fitted with a liquid fertiliser kit, running down the rear of each tine, to apply nutrients at five evenly spaced intervals to the foot of the tine. Ripping depth was set to 410mm, however the machine lifted across small areas of exceptionally hard, dry soil and rock within plots. With this three-point linkage machine there was no capacity to apply additional downward pressure on the ripper to prevent the rise.
See Parker et al (2020) for full methods of Beacon, Ongerup and Wyalkatchem trials where ripping treatments (depths) were applied with a modified Grizzly® Deep Digger, using both curved (parabolic) and straight shanked tines in a shallow leading tine formation. Ripping treatments at Beacon and Ongerup were applied during the autumn of 2015 using the shallow leading tine method. Wyalkatchem ripping treatments were implemented during summer 2017 using curved tines only. Beacon was ripped to a depth of 450mm, Wyalkatchem to 300mm while at Ongerup, Mingenew and Canna ripping was to 400mm. Topsoil slotting plates were used to bury surface soil, and surface applied nutrients of gypsum and composted chicken manure, to the depth of ripping, for rate see Parker et al (2020).
Nutrient treatments applied through the ripper at Mingenew and Canna include EzyFlow Nano Gypsum® at 0.652t/ha mined gypsum (23% Ca) equivalence, providing 115kgS/ha and 143kgCa/ha, and Urea Ammonia Nitrate (UAN) at 117L/ha to provide 37kg/ha of N. Rates were determined on discussion with industry experts and are slightly lower than McBeath et al (2010) 47kgN/ha. Gypsum was applied from the three lowest points on each tine, while Flexi-N was applied at each of the five outlets. Nutrients were applied to the surface through the ripper in non-ripped treatments. As gypsum and Flexi-N are not compatible they were applied separately requiring two passes of the ripper, with the ripper in the ground both times. This was achieved at Mingenew using real time kinematic (RTK) guidance to line the ripper with previous rip lines. At Canna these second passes were steered by hand.
The trials at Mingenew and Morawa were set out as randomised complete blocks and results were analysed using two-way ANOVA. Mingenew was sown with a trial plot seeder and knife point presswheel system while Canna was sown with the grower’s Ausplow DBS parallelogram system.
In-season fertiliser and herbicide applications were applied by the growers during whole of paddock operations.
Soil pits were dug and faced to expose roots on November 16 and 17, as soon after harvest as possible. Using a 50mm x 50mm mesh grid, scores of the number of roots were recorded for each cell within the mesh where 0 = 0 roots, 1 = 1-5 roots, 2 = 6-15 roots, 3 = 16-30 roots, 4 = >30 roots. For the sake of clarity in this paper the results are presented as a ‘heat map’ with greener colours representing the high scores graduating through yellow and orange to red for lower scores.
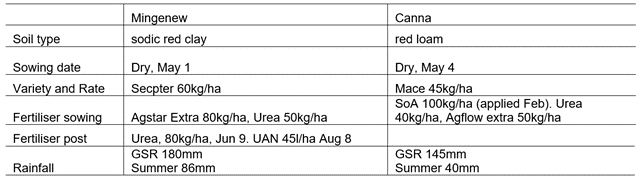
Table 1. Agronomic information from trial implementation.
Results
Soil test
Table 2. Nutrient levels in the soil profile (0–50 cm) from ripping trials conducted on clay soil. Colours indicate rating of nutrient, green – no concern, yellow – potential toxicity, orange – toxic levels. ESP: exchangeable sodium percentage, EC: electrical conductivity.
![]()
The amount of sodium increased with depth to toxic levels at Wyalkatchem, Beacon, Mingenew and Canna. Sodium toxicity, where exchangeable sodium percentage (ESP) was greater than 6%, occurred within the depth of ripping at each site. Beacon and Wyalkatchem are sites with clay percentages greater than 29% throughout the soil profile. Ongerup is a gritty duplex with a high percentage of clay at 10cm.
Emergence and yield
Table 3. Crop emergence and yield in various years since ripping was implemented. At Beacon and Ongerup ripping was implemented in 2015, at Wyalkatchem ripping was implemented in 2018 (all three trials conducted during project DAW00243), while at Mingenew and Canna ripping was implemented in 2020. TS: topsoil slotting, Gyp: gypsum, N: nitrogen. Crops w: wheat, b: barley, c: canola
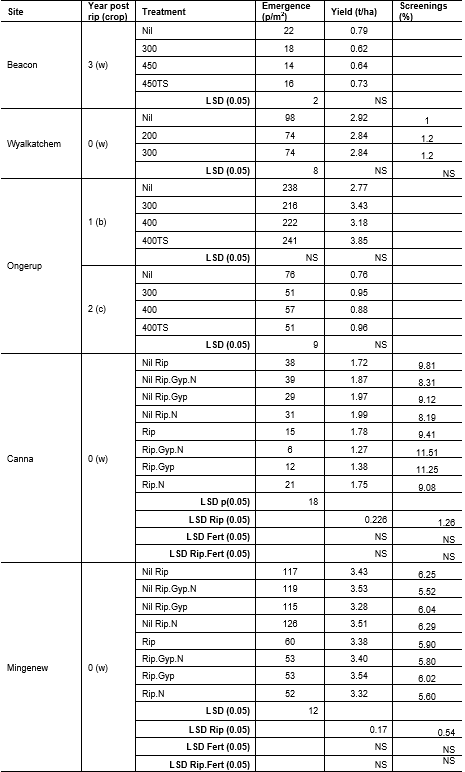
Emergence in the year of ripping (Year 0) was significantly reduced in the ripping treatments at Mingenew, Canna and Wyalkatchem (Table 3). Lower crop emergence continued in the years following ripping at Wyalkatchem (Parker et al 2020). At Ongerup canola had reduced emergence in the ripped plots two seasons after applying ripping treatments (Table 3), likely due to being a small-seeded species.
At Beacon there was no difference in yield between the control and ripped treatments three years after ripping. The same was true two years after ripping for barley at Ongerup where, in contrast to the results with canola, there was a trend of increased yield after ripping, though not significant due to site variability.
At Canna yield was reduced in the ripped treatments in the year of ripping (2020). At Mingenew, ripping with applied gypsum significantly increased yield compared to ripping alone. There was no difference in crop yield, at either site, between ripped and non-ripped where nutrient had been applied.
A lack of rain in September 2020 increased screening percentages to outside of receival standards at both Canna and Mingenew. Screenings at Canna were higher (3.2% more) in the ripping with gypsum and nitrogen treatments, but this was not significant. At Mingenew there was no difference in screenings between treatments. In contrast, at Wyalkatchem in the year of ripping (2018), screenings were not increased by ripping.
Root exploration assessment
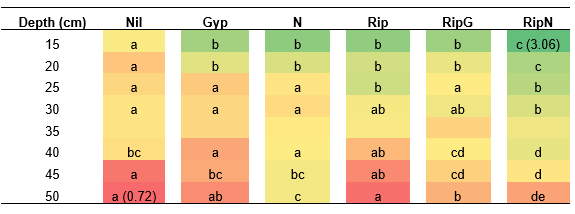
Nitrogen, with and without ripping, increased root numbers to depth at Canna (Table 4). There were additional roots in the top 20cm when compared to the control. Ripping with nitrogen inclusion increased root density throughout the profile when compared to non-ripped treatments (Table 4). Ripping increased root numbers to 25cm when compared to the control.
Table 4. Root density assessment of ripping and nutrient treatments at Canna. Colour is graded from green to red between highest and lowest result, where the darkest green is the highest average density score of 3.75, and the darkest red the lowest average density score 0.27. Comparing between treatments at the same depth different letters represent significance (p<0.05), at 35cm depth no letters are present as differences are insignificant at p<0.05. Gyp: gypsum, N: nitrogen
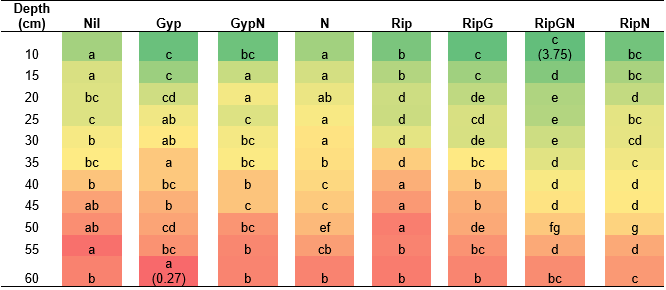
At Mingenew ripping with and without nutrients increased root density to 30cm (Table 5). Nitrogen did not increase root density as strongly as at Canna. Ripping with gypsum and nitrogen resulted in the greatest density of roots throughout the profile and to a depth comparable to rip plus nitrogen (55cm).
Table 5. Root assessment of ripping and nutrient treatments at Mingenew. Colour is graded from green to red between highest and lowest result, where the darkest green is the highest average root density score of 3.75, and the darkest red the lowest average root density of 0.27. Comparing between treatments at the same depth different letters represent significance (p<0.05). Gyp: gypsum, N: nitrogen
Conclusion
In the first year of trials at Mingenew and Canna deep ripping and deep nutrient placement increased the density of roots to a greater depth in sodic soil when compared to no ripping, either with or without additional nutrient. However, it is not known if this improved root growth and depth will remain and/or improve yield of future crops.
The efficacy of ripping is impacted by the nature of the season in which it is carried out. Seasons with hot dry finishes increase the occurrence of high screenings and small grain. In these sorts of seasons grain fill occurs as the soil profile is drying. Deep ripping is intended to increase the depth of soil that roots are able to access moisture from in order that deeper moisture might be used to fill grain. In 2020 there were no significant differences in screenings at Mingenew or Canna, despite more roots being found deeper in the profile where ripping and nutrients were applied together at both sites. It is likely that the pattern of rainfall and corresponding plant growth pattern ensured no deeper moisture was present when plants required it. August rainfall provide plant growth and yield potential that could not be met with the low September rain, 3mm and 8mm for Canna and Mingenew respectively.
Large clods often occur following deep ripping clay loam and clay soil. Soil clods can result in lower crop emergence in the seasons following ripping, as seen in these trials. Ripping was shown to increase root number at depth, but the reduced plant emergence means that crops grown on ripped soil may not have sufficient crop density to realise the yield potential provided by the ripping.
When ripping a sodic soil, it is best to leave toxic soil at depth to prevent damage of surface soil through dispersion and sodium toxicity. A slant tine ripper was selected to apply the ripping treatments at Mingenew and Canna because of its ripping action—a lift and drop action from the slanted leg, to shatter the soil. Soil rides over the leg with few soil clods lifted to the surface and hence the soil surface remains almost level and intact. By contrast, with straight leg rippers the heave from the tine is up and forward which pushes clods to the surface creating an uneven seed bed. With the slant tine ripper clods remained below the surface following the ripping pass. However, during seeding of trials at Mingenew and Canna the knife point of the seeder caught and lifted the buried clods, reducing the accuracy of seeding depth. The hand steered, second pass at Canna did not always align with previous rip lines and the ripper shank lifted previously fractured clods. Thus, it was the effect of this cloddiness, not sodicity, which reduced emergence and yield at Canna.
Deep ripping heavy soil must leave a paddock compatible with dry sowing if it is to have a positive outcome. This may require ripping during a fallow phase to allow natural consolidation of the soil and clods. Also required will be an increase in the sowing rate to compensate for lower emergence numbers in the cloddy soil. There is a need to reduce cloddiness, which can be achieved with shallow leading tine rippers or heavy crumble rollers, without reducing surface cover and increasing the risk of erosion.
Acknowledgments
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC and DPIRD, I would like to thank them for their continued support.
Our thanks to Katrina Sasse, Ben McTaggart and their respective families for hosting and managing the trial sites throughout a difficult 2020.
Thanks to Jo Walker, Chad Reynolds, Brandon Lauder and Melanie Kupsch for their assistance in the field when collecting data and to Dr. James Fisher for his review of the paper.
References
Hamilton, G., Fisher, P., Baimbridge, M., Bignell, J., Sheppard, J., and Bowey, R. (2005), Managing grey clays: to maximise production and sustainability. Department of Agriculture and Food, Western Australia. Bulletin 4666, 52p.
McBeath, T., Grant, C., Murray, R. and Chittleborough D (2010) Effects of subsoil amendments on soil physical properties, crop response and soil water quality in a dry year. Australian Journal of Soil Research, 48, pp 140-149.
Moore, G. (2004) Soil Guide; A handbook for understanding and managing agricultural soils. Department of Agriculture, Western Australia, Bulletin No. 4343.
Nuttall, J., Davies, S., Armstrong, R. and Peoples, M. (2008) Testing the primer-plant concept: wheat yields can be increased on alkaline sodic soils when an effective primer phase is used. Australian Journal of Agricultural Research, 59, pp 331-338.
Tavakkoli, E., Weng, Z., Tahmasbian, I., Uddin, S., Poile, G., Oates, A., Xu, B., Sandral, G., Fang, Y. and Armstrong, R. (2019) Understanding the amelioration processes of the subsoil application of amendments. Sourced from; https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2019/02/understanding-the-amelioration-processes-of-the-subsoil-application-of-amendments on 10/12/2020.
Contact details
Wayne Parker
Department of Primary Industries and Regional Development
20 Gregory st. Geraldton, 6530
Ph: 08 99568511
Email: wayne.parker@dpird.wa.gov.au
GRDC Project Code: DAW1902-001RTX, DAW1902-003RTX, DAW00243,
