Viral diseases in faba bean, chickpeas, lentil and lupins. Impacts, vectors/causes and management strategies for 2021
Author: Joop van Leur and Zorica Duric (NSW DPI, Tamworth Agricultural Research Institute) | Date: 24 Feb 2021
Take home messages
- The severe virus epidemic in faba bean in northern NSW during 2020 was initiated by early and massive flights of aphids (mainly cowpea aphids) that carried Bean yellow mosaic virus (BYMV) into the crops
- After a 2-year drought, heavy January and February rains in north-west NSW triggered the emergence of naturalised medics and other pasture legumes, which allowed a build-up of aphids and virus prior to the emergence of faba bean crops
- Most faba bean crops were sown into bare ground as cereal stubble was lacking after the extended drought. Lack of standing cereal stubble or uneven emergence makes pulse crops particularly vulnerable to early aphid infections
- Relatively mild and dry conditions during the start of the season favoured aphid multiplication in the faba bean crops and a fast spread of virus from initial infection foci
- Co-infections of BYMV and Alfalfa mosaic virus (AMV) caused particularly severe symptoms in several crops
- Both BYMV and AMV are non-persistently transmitted viruses that require only a short probing period by a viruliferous aphid to infect a plant. Slow-acting insecticides like imidacloprid applied to seed will not prevent infection of non-persistently transmitted viruses by incoming, winged, aphids . However, they may help in slowing the multiplication of aphids in the crop and subsequent spread of the virus by wingless aphids
- Further work is needed to investigate how long an imidacloprid seed dressing remains active and whether a foliar application of a pulse registered product (pirimicarb) after aphids are found in a crop is more practical, economic or effective
- There are no indications that transmission of BYMV to faba bean seed occurs at significant levels
- Virus control strategies are all based on preventing infection, particularly during the early growth stages of the crop. Poorly emerging crops or crops sown into bare ground are particularly vulnerable to infection. Sowing in standing cereal stubble and using high quality seed with good seedling vigour has shown to be the most reliable management option to avoid virus infections.
Pulse viruses and pulse virus vectors
Pulses are far more vulnerable to virus infection than winter cereals with over 20 viruses considered to be of economic importance on pulse crops worldwide. All major pulse viruses require an insect vector (mostly an aphid species) to transmit the virus to a healthy plant. The mode of transmission can be non-persistent, which means that the aphid only needs a short probe of the plant in order to acquire or to transmit the virus, while persistently transmitted viruses require a longer feeding period by the aphid. Plants can be infected by most non-persistently transmitted viruses under experimental conditions through rubbing a virus suspension into the leaf (‘mechanical inoculation’), while a viruliferous vector is always needed for the persistently transmitted viruses. Viruliferous aphids will lose a non-persistently transmitted virus after probing a healthy plant, but aphids that carry a persistently transmitted virus remain viruliferous.
The difference in the time needed to transmit a virus has implications for the effectiveness of aphicides to control virus infections; infection by a persistently transmitted virus like Bean leafroll virus (BLRV) can be prevented by a timely foliar application of an insecticide or by seed dressing with a systemic insecticide, but most aphicides are not fast enough to prevent infection by a non-persistently transmitted virus like Bean yellow mosaic virus (BYMV).
Virus epidemiology and the prediction of severe virus epidemics is complicated as aphid numbers and movements depend on numerous factors, both during and preceding the growing season. A few viruses can survive in seed, but most require a ‘green bridge’ of living host to survive between cropping seasons. Pulse viruses are not host species specific and naturalised pasture legumes, like medics or sub clovers, or perennial legumes, like lucerne or white clover, can be infected with a range of viruses that can be harmful to grain legumes like faba bean or chickpea. Viruses like BYMV and AMV can be seed transmitted in several pasture legume species and viruses and their vectors can build up in pastures, roadside weeds or stock routes prior to the emergence of pulse crops. Also, a virus that can devastate grain legumes might have little or no impact on a pasture legume or a weed; e.g. Bean leafroll virus (BLRV) kills pulses like faba bean, field pea, lentil and field pea, but is symptomless on lucerne, its main summer host.
Different aphid species have different temperature optima, but generally mild weather favours aphid multiplication. Aphids can multiply very fast and host plant population can become rapidly overcrowded, particularly if the host plants are still in an early stage of development (as with naturalised pasture legumes during late summer-early autumn). Overcrowding or deterioration of the host plant will trigger the development of winged aphids who can, largely through wind currents, migrate over long distances. Large peaks in movements of winged aphids can be observed during autumn and can be harmful for early sown crops like faba bean and lupins. Aphid activity generally slows down during winter but picks up again with rising temperatures and major aphid flights can be again observed in spring. Later-sown pulse crops like chickpeas generally escape the autumn aphid flights but are vulnerable to the spring flights.
Faba bean viruses in northern NSW
Within Australia 12 viruses have been reported to infect faba bean, but not more than five have caused serious yield reductions in the past (Table 1). Surveys in northern NSW over the last two decades identified Bean leafroll virus (BLRV) as the most important faba bean virus. Severe symptoms, like stunting and plant death, were found after infection by BLRV and (less frequently) by the closely related Soybean dwarf virus (SbDV). Heavy yield losses by BLRV were recorded in the early 2000’s on the highly susceptible cultivar Fiord, particularly in paddocks close to lucerne, the major BLRV summer host. Germplasm screening for resistance was successful in identifying good sources of BLRV resistance, which were used in the breeding program to develop varieties with improved resistance.
In 2004 and 2005 high incidences of the thrips transmitted Tomato spotted wilt virus (TSWV) were found in faba bean paddocks north of Moree. The sudden occurrence of TSWV was likely a result of the recent incursion of the western flower thrips (Frankliniella occidentalis), a highly effective vector. TSWV has a very wide host range and is lethal on faba bean. However, although infections of up to 10% were found in some crops, infected plants remained randomly scattered through paddocks and, surprisingly, no secondary spread could be noticed. TSWV was rarely found in more recent surveys. Other viruses that can cause plant death, but only occur sporadically, are Sub clover stunt virus (SCSV) and Clover yellow vein virus (CYVV).
Table 1. Faba bean viruses in Australia (in alphabetical order)
Virus | Abr. | Transmission | Major vectors | Seed transmission1 | Occurrence in NSW faba bean crops | Impact on plants |
|---|---|---|---|---|---|---|
Alfalfa mosaic | AMV | non-persistent | many aphid species | medics, lucerne, lupin | low incidences every year | moderate |
Bean leafroll | BLRV | persistent | pea, cowpea aphid | no | very high occasionally | high |
Bean yellow mosaic | BYMV | non-persistent | many aphid species | medics, sub clover, (faba bean) | high incidences regularly, normally at end of season | high after early infection |
Broad bean wilt | BBWV | non-persistent | green peach aphid and others | no | very rare | high |
Cucumber mosaic | CMV | non-persistent | many aphid species | lupin, lentil | very rare | low |
Clover yellow vein | CYVV | non-persistent | cowpea aphid | no | rare | high |
Pea seed-borne mosaic | PSbMV | non-persistent | many aphid species | field pea, (lentil) | rare | low |
Phasey bean mild yellows | PBMYV | persistent | cowpea aphid | no | ? | ? |
Sub clover stunt | SCSV | persistent | cowpea aphid | no | generally rare, widespread occasionally | high |
Soybean dwarf | SbDV | persistent | pea aphid and others | no | low incidences regularly | high |
Turnip yellows2 | TuYV | persistent | green peach aphid | no | high incidences regularly at end of the season | moderate |
Tomato spotted wilt | TSWV | persistent | Western flower thrips and other thrips | no | occasionally low incidences | high |
1 Seed transmission of host species listed between brackets is reported, but unlikely to be of significance
2 TuYV was previously known as Beet western yellows virus (BWYV)
While survey results indicated that BLRV was the most important virus because of its impact on yield, BYMV was always the most frequently identified virus throughout northern NSW. Generally, infections appeared late in the season and symptoms consisted only of a relatively mild mosaic in the top leaves above the pod setting nodes and no serious impact on yield was noticeable in commercial crops. However, yield loss trials comparing BYMV inoculated with non-inoculated plots (using mechanical inoculation at the 3-4 leaf stage) demonstrated that early infections could have a major impact on yield with over 50% yield reduction on PBA Warda and PBA Nasma (the two most common varieties in northern NSW) and close to 70% yield reduction on the highly susceptible southern variety Fiesta VF. Large numbers of breeding lines and germplasm accessions were evaluated in BYMV inoculated screening trials over the years, but no lines with high levels of resistance were identified. Unlike with BLRV, no plant death was observed in the yield loss or resistance screening trials, except for several germplasm accessions from Ecuadorian origin that showed hyper-sensitive reactions.
The 2020 faba bean virus epidemic in northern NSW
By early June 2020 serious symptoms in early sown faba bean crops were reported in several sites in northern NSW. Patches of necrotic and stunted plants in the affected paddocks resembled severe early infections by BLRV, SCSV or TSWV seen in earlier years. However, testing of symptomatic plants by Tissue Blot Immunoassay (TBIA) at the Tamworth Agricultural Institute (TAI) and by molecular tests at the QDAF virology laboratory in Brisbane (Dr Murray Sharman) showed the main virus to be BYMV, with co-infection by AMV in some paddocks (Table 2).
Table 2. Presence of three major faba bean viruses and one virus group in collected and submitted faba bean samples, northern NSW, 2020 1
Symptom type | Tested plants | % positive plants as determined by TBIA | |||
|---|---|---|---|---|---|
BYMV | AMV | BLRV | Luteovirus2 | ||
Randomly collected | 359 | 44.3 | 13.1 | 1.1 | 7.5 |
No symptoms | 407 | 49.9 | 6.4 | 0.2 | 2.0 |
Not specified virus symptoms | 279 | 58.4 | 15.4 | 2.5 | 9.0 |
Plant necrosis | 329 | 81.8 | 26.4 | 0.3 | 2.4 |
Plant stunting | 194 | 66.0 | 5.2 | 12.9 | 7.2 |
Leaf mosaic | 234 | 88.9 | 11.5 | 0.0 | 3.8 |
Total plants tested | 1802 | 62.7 | 13.3 | 2.1 | 5.0 |
1 Minor incidences found for SCSV (7 plants out of 325 tested), CYVV (1 plant out of 991 tested) and Cucumber mosaic virus (CMV, 4 plants out of 682 tested)
2 Luteoviruses other than BLRV
The early infections followed high populations of migrating aphids observed on aphid traps during autumn 2020 (Figure 1). The main aphid species found on the traps was cowpea aphid (Aphis craccivora), but other aphid species known to colonise pulse species were also found in high numbers: pea aphid (Acyrthosiphon pisum), blue-green aphid (Acyrthosiphon pisum) and green-peach aphid (Myzus persicae). Cowpea aphid is a highly effective vector of both persistently and non-persistently transmitted viruses, and - equally important - it colonises and multiplies on faba bean. Further surveys and testing during the season showed clear foci spreading from early infected plants, a pattern typical of colonising, wingless aphids.
Several agronomists expressed concern that green mirids (Creontiades dilutes), which were in abundance in faba bean crops during 2020, were capable of transmitting BYMV. We have undertaken two separate trials in which green mirids were left for 24 hours on BYMV infected faba bean plants in insect proof tents, before healthy faba bean plants were placed in the same tent. No BYMV transmission from the infected to the healthy plants was observed, confirming numerous publications that only aphids can transmit this virus.
As the season progressed the virus spread through most faba bean paddocks in northern NSW, but severe symptoms generally remained restricted to early infected paddocks. Large differences in severity could be observed between paddocks that were at close distance, and paddocks close to the Queensland border and those south of Gilgandra were less affected (Figure 2). Virologists from QDAF confirmed the presence of BYMV in faba bean paddocks in Queensland, but severe symptoms were rare except for an irrigated crop near St George.
 Figure 1. Aphids (number of aphids / m2 yellow sticky trap / day) caught on yellow sticky traps in 5 sites in northern NSW, Autumn 2020
Figure 1. Aphids (number of aphids / m2 yellow sticky trap / day) caught on yellow sticky traps in 5 sites in northern NSW, Autumn 2020
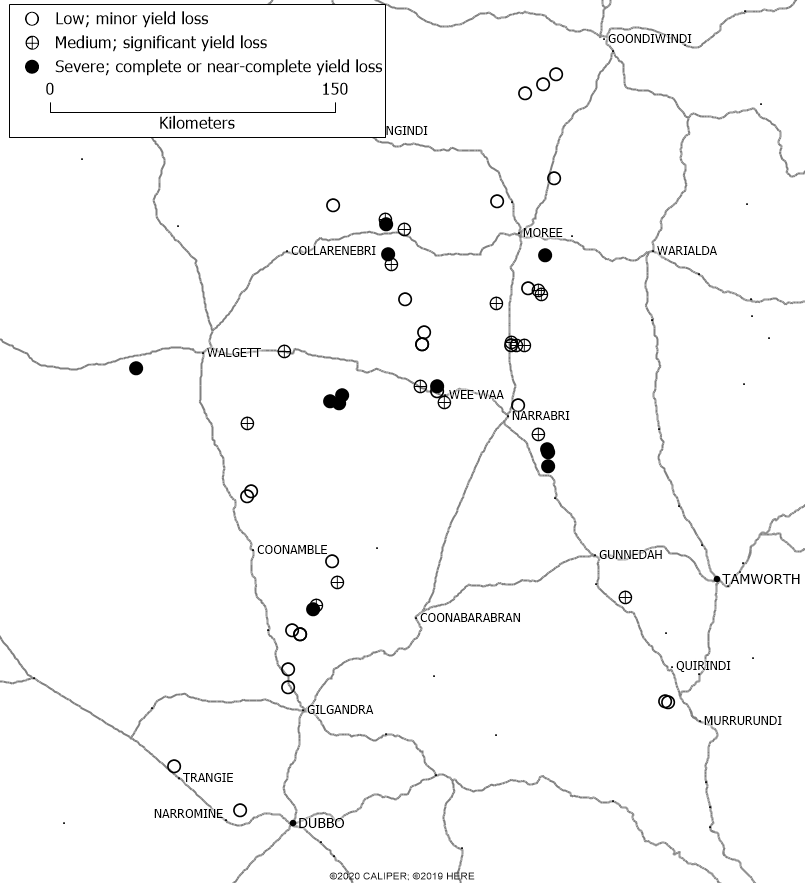 Figure 2. Severity of virus infections in 55 faba bean paddocks Northern NSW, 2020
Figure 2. Severity of virus infections in 55 faba bean paddocks Northern NSW, 2020
The very high early aphid numbers and severe virus in north-western NSW points to a possible role of naturalised pasture legumes in the epidemiology of pulse viruses: After two very dry years, the January - February rains in the north-western part of the state would have triggered the emergence of large areas of medics and other potential virus hosts. Mild temperatures promoted a fast multiplication of aphids and when aphid populations on the medics became too crowded, winged aphids would have moved to newly emerged faba bean crops. BYMV (and AMV) are both transmitted in medic seed, so even with low seed transmission rates enough aphids could have picked up inoculum to establish infection foci in faba bean crops. Continuing mild temperatures during the start of the season allowed multiplication of aphids in the faba bean crops and spread of the virus from the initial infection foci. The preceding drought also had indirect effects on the high level of virus infections: Aphids tend to avoid crops sown in standing cereal stubble and with less cereal crops grown in 2019, more faba bean crops were sown into bare ground. Also, faba bean seed was in short supply and some seed used was rather old and less vigorous.
While the unusual severity of BYMV infection during 2020 was likely a direct result of very early infections and, in some cases, co-infection by AMV, it is also possible that more virulent BYMV strains were present. BYMV is known to be variable in pathogenicity and host species specificity. We have isolated a large number of strains this year and are in the process of evaluating these using pathogenicity testing and molecular tools.
Viruses in crops other than faba bean
Virus symptoms were observed in several chickpea crops in the Moree-Narrabri region that were bordering faba bean paddocks. The level of infection was high close to the border but diminished rapidly further from the faba bean crop. Testing of virus symptomatic plants showed that while the faba bean paddocks had high BYMV and minor AMV infection levels, symptomatic chickpeas were only infected by AMV. BYMV is reported to be capable of infecting chickpeas, but in our greenhouse tests chickpeas reacted as immune to the majority of BYMV strains isolated from faba bean.
Random samples taken from chickpea paddocks by Dr Kevin Moore as part of his yearly disease survey (Table 3), showed that the later sown chickpea crops generally escaped the aphid autumn flights. The absence of virus spread in chickpea paddocks also demonstrated that aphids rarely colonise chickpeas and infection is generally caused by incoming, winged aphids.
Table 3. Presence of four major pulse viruses and one virus group in collected and submitted samples of pulses (other than faba bean), NSW, 2020.
Species | Tested plants | % positive plants as determined by TBIA | ||||
|---|---|---|---|---|---|---|
BYMV | AMV | BLRV | Luteovirus1) | CMV | ||
Chickpeas | 1,329 | 0.0 | 5.5 | 0.3 | 1.1 | 1.1 |
Lentils | 1,161 | 0.2 | 12.7 | 2.7 | 0.3 | 34.6 |
Lupins | 227 | 1.3 | 28.6 | 0.9 | 0.0 | 90.3 |
1 Luteoviruses other than BLRV
Both lupins and lentils are, unlike chickpeas, crops colonised by several aphid species and highly vulnerable to infection by a range of viruses. CMV caused severe symptoms in narrow-leafed lupin crops in the Gilgandra region with co-infection by AMV aggravating losses in several paddocks. CMV can be seed-transmitted in narrow-leafed lupins at high rates and testing of several seed lots that were used for the 2020 sowing showed dangerously high CMV levels. Narrow-leafed lupins in NSW are generally used as stockfeed on farm and most growers multiply their own seed stock for several years. While virus infections might remain unnoticed in most years, seed infection levels can build up slowly and cause a severe virus epidemic in an aphid favourable season like 2020.
Lentils are still an experimental crop in most of NSW, but the high levels of CMV and other viruses in lentil samples taken from trial sites (Table 3) showed that viruses may become a major factor limiting the expansion of lentils.
Virus control strategies
Virus control strategies will differ between crops and virus species, but all are aimed at avoiding infection as curative control of viruses is not possible.
Minimising numbers of incoming virus vectors
The most effective strategy currently is to minimise virus infection by promoting fast canopy closure through following optimal agronomic practices and use of high quality seed with good seedling vigour. Migrating aphids prefer landing in crops that show uneven emergence and bare ground, while sowing in standing stubble deters aphid landings. Early sowing increases the risk of virus infection as crops are exposed to the autumn aphid flights when plants are small and most vulnerable to virus infection. However, later sowing is often not a practical option for growers.
Virus-free seed
Several non-persistently transmitted viruses can be seed-borne in pulses. Sowing virus-infected seed will result in infection foci scattered randomly throughout a crop right after crop emergence and a rapid spread of the virus. Fortunately, for most virus / pulse host combinations, the levels of seed transmission are very low and, while still very important for quarantine, not of concern in commercial crops. The exceptions are CMV in narrow-leafed lupins and lentils and Pea seed-borne mosaic virus (PSbMV) in field peas. Growers who keep their own seed should have it tested and only use virus-free seed.
BYMV can be seed-transmitted in faba bean, but so far we haven’t found any evidence of its presence in commercial or farmers’ seed lots. However, new virus strains can develop that have a better ability to be seed transmitted, so our testing program is continuing.
Avoiding inoculum sources
Unlike most fungal pathogens, viruses don’t survive in stubble or soil. Apart from those that are seed-transmitted all will be brought into the crop by vectors from outside sources. It is advisable to keep distance from known sources of infection like lucerne. Weed and volunteer legume crops within or near crops are also key virus sources and should be controlled before the crop emerges.
Chemical control of vectors
The effectiveness of aphicides to control virus infection differs between viruses. Viruses that are persistently transmitted, like BLRV or TuYV, require a relatively long feeding period and a seed treatment with a systemic insecticide can provide protection during early growth when plants are most vulnerable. Non-persistently transmitted viruses, like BYMV, CMV or AMV, only require a brief probing to transmit virus and the insecticides used for seed treatment act too slowly to prevent infection.
Imidacloprid is registered as a seed treatment for early aphid control on faba bean, field pea and lentils. The seed treatment will not stop initial BYMV infections from migrating aphids, but it could possibly delay the build-up of colonising aphids in a crop and thereby limit virus spread. Greenhouse trials in 2020 confirmed that imidacloprid seed treatment reduced the multiplication of cowpea and pea aphid numbers on faba bean, but it took more than one day to kill aphids. Field research is needed to determine how long an imidacloprid seed treatment remains effective and whether it will be more useful than a well-timed foliar aphicide application.
Resistance
Genetic resistance can be a very economical and environmentally friendly option for virus control. Over the years good resistance has been identified for several pulse / virus combinations and subsequently used in Australian pulse breeding programs.
Unfortunately, the search for high levels of BYMV resistance in faba bean has not been successful so far. There are differences among Australian faba bean varieties in symptom expression with some of the older varieties and lines developed for the southern region reacting as ‘very susceptible’. There were no indications that the current northern region varieties differed in their BYMV reaction during the 2020 epidemic, but a few breeding lines yielded well in different sites under severe BYMV pressure. These lines will be tested during the 2021 season in inoculated trials.
All narrow-leafed lupin varieties are CMV susceptible, but a number of varieties are moderately resistant to CMV seed infection. While these varieties are still susceptible to CMV by aphid transmission, the build-up of inoculum in seed stock will be slower and the risk on CMV induced losses during virus favourable seasons will be lower.
References
There is a wealth of information on pulse viruses published on the internet by the agricultural departments of Western Australia, Queensland, Victoria and New South Wales.
For more detailed information:
Makkouk KM, Kumari SG, van Leur JAG, Jones RAC (2014). Control of plant virus diseases in cool-season grain legume crops. Advances in Virus Research 90: 207-253.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support.
We would also like to acknowledge co-investment in this project by GRDC and NSW DPI through the Grains Agronomy Pathology Partnership (GAPP) and the outstanding collaboration and useful discussions with growers and agronomists during surveys.
Contact details
Joop van Leur
NSW Department of Primary Industries
4 Marsden Park Rd, Tamworth, 2340, NSW
Ph: 0427 928 018
Email: joop.vanleur@dpi.nsw.gov.au
Zorica Duric
NSW Department of Primary Industries
4 Marsden Park Rd, Tamworth, 2340, NSW
Ph: 02 6763 1154
Email: zorica.duric@dpi.nsw.gov.au
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
GRDC Project Code: DAN00202, DAN00213/BLG209, DAN00213/BLG204,
Was this page helpful?
YOUR FEEDBACK
