Fall armyworm update
Author: Dr Melina Miles (Queensland Department of Agriculture and Fisheries) and Dr Lisa Bird (NSW Department of Primary Industries) | Date: 02 Mar 2021
Take home messages
- Fall armyworm (FAW) is now widespread in all growing regions of the northern grains region
- Monitoring of susceptible crops including maize (for grain or silage), and sorghum is now essential at crop establishment and vegetative stages. Monitoring from tasselling and head emergence in these crops should now include FAW
- Natural enemies are very active on FAW and it is important to look for them when checking crops
- Recent testing has revealed established insecticide resistance in Australian FAW populations. A planned approach to insecticide selection and rotation is required to minimise further resistance development and optimise the cost-effectiveness of insecticide applications.
Fall armyworm resistance screening research
Reliance on chemical control in the management of FAW on a global scale over many decades has led to the development of resistance to at least 29 insecticide active ingredients in six mode-of-action groups.
To understand how this will impact the effectiveness of chemical control options, NSW DPI with support from the CRDC, has conducted research to establish the toxicity profiles of insecticide groups currently registered under permit for FAW in Australia (Table 1). This information will assist growers in deciding the most appropriate course of action for managing outbreaks of FAW if sprays are warranted.
The screening procedures were adapted from those previously developed for laboratory-based resistance testing in Helicoverpa. In the case of broad-spectrum contact insecticides (such as synthetic pyrethroids and carbamates), testing was done by topical application of insecticide as a backline treatment to FAW larvae. On the other hand, selective insecticides (such as the Group 28s, indoxacarb, spinosyns and avermectins) are more active by ingestion than by contact. Therefore, these insecticides were administered by adding formulated insecticide to an artificial diet on which the insect larvae were fed. Since all insecticides are also registered for Helicoverpa armigera (cotton bollworm), the dose response in H. armigera was used as a standard for comparing the relative efficacy of products in FAW.
The key findings from the research are;
- A similar level of toxicity (efficacy) of emamectin benzoate (e.g. Affirm®) and Group 5 insecticides spinosad and spinetoram (e.g. Entrust® and Success Neo®) in both H. armigera and FAW at all levels of the dose response.
- A similar level of toxicity (efficacy) of the Group 28 insecticide chlorantraniliprole (e.g. Altacor®) at high doses in H. armigera and FAW. However, FAW was about 2 times less sensitive at the median lethal concentration (LC50) of chlorantraniliprole.
- The toxicity of indoxacarb (e.g. Steward®) was significantly lower in FAW compared with H. armigera and probably represents a naturally higher tolerance to indoxacarb in FAW.
- There was a small but significant reduction in sensitivity to methomyl in FAW larvae compared with H. armigera. This is consistent with the detection of genetic markers for carbamate resistance in FAW. However, moths of FAW remain fully susceptible to methomyl.
- FAW is 50-80 times less sensitive to the synthetic pyrethroids (SP) alpha-cypermethrin and gamma-cyhalothrin compared with susceptible H. armigera. Therefore, based on our experience with H. armigera with similar levels of SP resistance, it is highly unlikely that field rates of these insecticides will control FAW, even under optimal spray conditions.
- There is strong evidence to suggest metabolic (not target site) resistance to SP in FAW. Metabolic resistance is an important mechanism which is also known to confer very high levels of SP resistance in H. armigera.
Table 1. Summary of permits for fall armyworm control as of January 2021.
Active Constituent | MOA Group | Permit number |
|---|---|---|
Methomyl | 1A | PER89279, PER89293, PER89400, PER89330 |
Alpha-cypermethrin | 3A | PER89279, PER85447, PER89425, PER89330 |
Gamma-cyhalothrin | 3A | PER89358 |
Spinetoram | 5 | PER89241, PER89331, PER89327, PER89284, PER89390 |
Spinosad | 5 | PER89870 |
Emamectin benzoate | 6 | PER89285, PER89263, PER89300, PER89344, PER89371, PER89330 |
Indoxacarb | 22A | PER89306, PER89279, PER89278, PER89311, PER89530, PER89286, PER90374, PER89330 |
Chlorantraniliprole | 28 | PER89290, PER89366, PER89281, PER89353, PER89384, PER89259, PER89354, PER89457, PER89330 |
Implications for management
High levels of metabolic resistance to SP and presence of genetic markers for resistance to carbamates and organophosphates indicate that broad-spectrum insecticides are unlikely to provide effective control of FAW. Given the levels of resistance to broad-spectrums, growers are strongly advised to avoid using these chemical groups and instead consider adopting IPM strategies which help optimise the cost of controlling FAW by taking advantage of natural enemies present in crops.
High levels of susceptibility to selective insecticides such as emamectin benzoate and spinetoram indicate these insecticides will be effective options for management. The Group 28 insecticide chlorantraniliprole is also likely to provide effective control. However, control may be marginal at rates below the full field rate of this insecticide.
A natural tolerance to indoxacarb in FAW suggests this insecticide may not provide effective control in crops with high insect pressure. However, indoxacarb may be useful for achieving population suppression in low pressure situations and for providing an additional rotation option for resistance management.
As with any insect pest, there is considerable potential for further selection of resistance in FAW to selective insecticides if usage increases. Overuse of selective insecticides could also threaten Helicoverpa resistance management if there is an increase in the frequency of sprays in crops where the two species occur together.
As a first step toward pre-emptive management of resistance to pivotal selective insecticides used in the management of FAW, NSW DPI has developed resistance screening procedures to increase capacity for detecting field resistance in FAW. Diagnostic concentrations of indoxacarb, chlorantraniliprole, emamectin benzoate and spinetoram have now been established for detecting future changes in resistance to these insecticides which will be a critical component of future resistance management strategies that are developed for FAW.
Although there is currently no resistance management strategy (RMS) for FAW, the key principles of resistance management and IPM should be applied when making spray decisions. This includes regular monitoring to identify early outbreaks, timely applications of selective insecticides on above threshold populations, and rotating selective insecticides with different modes of action.
Following these guidelines will help to optimise the cost of applications and control of FAW while making the most of natural enemy populations present in the crop, the benefits of which will be destroyed by broad-spectrum insecticides that are unlikely to provide effective control.
Detecting FAW in sorghum and corn
FAW are active across the northern grains region, but inland central and southern Qld have not experienced continuous population build up in crops since the first immigration of moths in September – October. Infestations are typically patchy across fields with ‘hotspots’ of heavily impacted plants. Evidence of FAW infestation appear at any stage of crop growth, but no reports of significant crop loss have been recorded to date.
One of the major contributors to this continuing low pressure and minor crop damage is probably the very high natural enemy (beneficial) impact on FAW. There are a number of common parasitoids and predators we are familiar with as natural enemies of helicoverpa, armyworms and loopers that are being observed attacking FAW. These include the larval parasitoid wasp Cotesia sp. (possibly Cotesia ruficrus) and the spined predatory bug (Oechalia schellenbergii). One of the other common, but more difficult to see, natural enemies is Trichogramma (egg parasitoid). In north Queensland, Trichogramma egg parasitism has been very common in our trial plots. When Trichogramma find a FAW egg mass, all eggs are parasitised, or at least the vast majority.

Figure 1. The most commonly observed natural enemies attacking FAW to date. (Left to right). Group of white, fluffy pupal cocoons of the larval parasitoid, Cotesia sp. The spined predatory shield bug making a meal of a FAW larva. A FAW egg mass that has been parasitised by Trichogramma. The small exit holes in the eggs are visible where the wasps have emerged.
Larval identification basics
It is very likely that both helicoverpa and FAW will be present in most maize and sorghum crops, as well as native armyworm (common, northern) in some crops. There are identification resources on the Beatsheet.
FAW and helicoverpa are very similar. Once you have had a bit of experience with FAW, the differences will be obvious, but until then it is a struggle to work out which is which, and why. These images below have been sent in for checking – to see if they are FAW or helicoverpa. If in doubt, please to send photos to Melina Miles (Melina.Miles@daf.qld.gov.au) or Hugh Brier (hugh.brier@daf.qld.gov.au).
The annotation in Figure 2 (excuse the amateur attempt) identifies the features that determine whether they are helicoverpa or FAW. As with all insects and characters, there is variation between individuals. Darker FAW larvae can look to have darker spots etc. In the case of Figure 2 below, this is a Helicoverpa armigera.
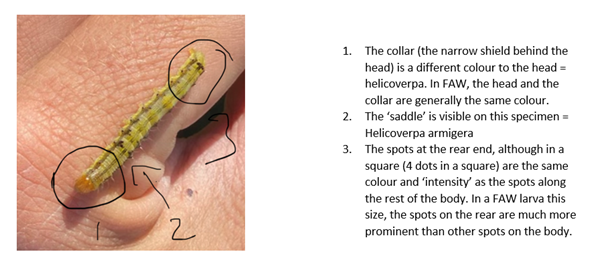
Figure 2. Identification points differentiating Helicoverpa armigera and fall armyworm (picture is Helicoverpa armigera)

Figure 3. Helicoverpa and FAW larvae (3rd-4th instar) are very difficult to tell apart, until you have had some experience with them. These images illustrate how similar the two are at this stage. Helicoverpa (left image), FAW (right).

Figure 4. Large FAW larvae, like this one, start to look greasy and less hairy than younger FAW or helicoverpa. In this image you can see how much darker and more raised the square of spots is on the rear end. This is very characteristic of late instar FAW.
Recognising FAW damage
What will be most obvious, when FAW are present, is the amount of damage that they cause to the leaves. We are used to seeing some windowing from helicoverpa, but largely they do very little damage to the developing whorl. The worst of it is usually ‘shot holes’ in the expanded leaves (see Figure 5).

Figure 5. (Left) Helicoverpa damage to sorghum tends to be minor windowing evident in expanded leaves. The whorl is largely unaffected. Photos: Rory Kerlin. (Right) Shotgun holes in leaves, typical of helicoverpa damage.
In contrast, FAW larvae feeding in the whorl cause significant damage to the whorl. This will be evident, not only by examining the whorl, but from the large holes in the expanding leaves as a result of the amount of damage caused in the whorl (Figure 6)

Figure 6. FAW damage to sorghum, showing the extensive damage to the whorl, and the large holes evident as leaves expand. The last image shows a sorghum plant with very characteristic damage to the whorl where the terminal end of the whorl has been chewed off. As a result, the whorl and emerging leaves are cut (or break) off flat.
FAW larvae causing extensive damage in the whorl are generally medium and large larvae. Smaller larvae can be present in the whorl, and unrolling the whorl may expose multiple larvae that have segregated themselves from each other in the different layers.
Feeding by very small and small FAW larvae that have recently emerged from eggs (windowing) is also an indication of FAW activity in the crop. Keep in mind that windowing will persist on leaves long after the larvae have moved to the whorl or died. With experience, new and old feeding damage can be distinguished (Figure 7).

Figure 7. (Left) Feeding damage caused by newly hatched FAW larvae. The remains of the egg mass (moth scales) is just visible on the RHS of the feeding damage. (Right) Windowing caused by small FAW not yet established in the whorl.
The other thing you will notice with FAW, that you don’t see so much with helicoverpa, is the amount of poo/frass they produce. Huge amounts of it that fills the whorl. It is wet and sloppy, not dry pellets like helicoverpa and native armyworm species tend to produce (Figure 8).

Figure 8. Comparison of frass (poo) of helicoverpa and FAW. Helicoverpa poo (left) is dry. FAW poo is sloppy and messy, and there is a lot of it! (right).
FAW moths are often sighted resting in crops, in similar locations to Helicoverpa moths (Figure 9). Both male and female moths have been observed resting in whorls particularly.

Figure 9. Female FAW moth.
Scouting for eggs can feel like looking for a needle in a haystack. There is so much leaf area in a crop of sorghum or maize. Our experience in north Qld so far, has been that eggs are often laid in about the same place on plants. On small plants, they are often on the undersides of the leaves, towards the base (Figure 7). Eggs are not always covered in fluffy scales from the moth as in Figure 10a and 10b. Some are covered in scales like the egg mass in Figure 10c. We have found it a bit easier to find eggs on the underside of leaves if you are checking in the early morning or late afternoon. At these times of the day the eggs on the underside of the leaf can be silhouetted (Figure 10d), meaning that you can walk along rows looking for the shadows, rather than turning leaves over constantly.

Figure 10. Location of FAW eggs are laid on the underside of leaves in young crops.
Damage to establishing crops can include plant death
In establishing crops, FAW will not feed only on leaves. They can also burrow into the base of young plants, resulting in the death of the plant. Where we have seen this happen it has been where there are large larvae which have consumed most of the plant and then taken shelter during the day in the soil. These large larvae will sit under the soil surface feeding on the base of the seedling plants (Figure 11). For this reason, it is important that emerging crops are checked at least weekly. If a high percentage of plants have FAW larvae, and the larvae are medium or larger, there is a risk that they could start to do this kind of damage. Once plants have 6-8 leaves, there is more leaf and less likelihood of this type of damage occurring.

Figure 11. Large FAW larvae sheltering under the soil surface and feeding on the base of seedlings. Damage caused by this feeding. Larvae can burrow up inside the stem too.
If the damage observed in a young crop seems disproportionately high for the number, or size, of larvae being observed on plants, check for large larvae around the base of plants. It is possible that large, very damaging larvae, are moving up onto plants at night to feed, but are not there to be sampled during the day.
References
The Beatsheet has resources on FAW and other caterpillar identification, pheromone trap catch data for Qld and NSW, webinars, access to a development model for insect pest species, how-to videos and regular updates on regional pest issues.
APVMA PUBCRIS database (search for fall armyworm) to find current permits for control options.
Acknowledgements
CRDC, NSW DPI and the Queensland Government have provided financial support for the R, D and E presented in this paper. We also thank agronomists and growers who have provided FAW collections for resistance testing and/or provided access to crops on their farms, and to the QDAF farm staff at the Ayr Research Station.
Unless photographer is specifically identified, all photographs by Melina Miles.
Contact details
Dr Melina Miles
Department of Agriculture and Fisheries, Toowoomba
Ph: 07 45294169 / 0407113306
Email: melina.miles@daf.qld.gov.au
Dr Lisa Bird
NSW Department of Primary Industries, Tamworth
Ph: 02 67631128 / 0438623906
Email: lisa.bird@dpi.nsw.gov.au
® Registered trademark
Was this page helpful?
YOUR FEEDBACK
