Herbicide resistance and optimising glyphosate - Dalby
Herbicide resistance and optimising glyphosate - Dalby
Author: Peter Boutsalis (School of Agriculture, Food & Wine, University of Adelaide & Plant Science Consulting) & Christopher Preston (School of Agriculture, Food & Wine, University of Adelaide) | Date: 02 Mar 2021
Take home messages
- Glyphosate performance can be optimised through attention to application variables such as temperature, plant stress and formulation.
- Wild oat resistance levels show effective herbicide options are available.
- Resistance testing is important to identify effective herbicides.
Evolution of glyphosate resistance
Glyphosate was first registered in the 1970’s and rapidly became the benchmark herbicide for non-selective weed control. The first weed species to develop resistance to glyphosate was annual ryegrass that occurred in an orchard in southern NSW (Powles et. al. 1998). Only a few cases of resistance were detected in the following decade . The fact that it required decades of repeated use before resistance to other species was confirmed indicated that the natural frequency of glyphosate resistance was initially very low. At the current time there are over a dozen species that have developed resistance to glyphosate in Australia. The most important species are ryegrass, awnless barnyard grass, feathertop Rhodes grass, liverseed grass, flaxleaf fleabane and sowthistle.
Resistance is generally not ’black and white‘ with resistance levels differing between populations of resistant weeds and even between individuals of the same population. Differences in the level of resistance have been detected in barnyard grass (Figure 1).

Increasing glyphosate resistance
There are several contributing factors for the increasing incidence in glyphosate resistance, with generally more than one factor responsible. These include:
- Reducing rates or using poor quality formulations, resulting in sub-lethal control (Figure 2)
- Mixing glyphosate with too many other active ingredients resulting in antagonism, particularly in low water volumes
- Using low quality water, particularly hard water. Glyphosate is a weak acid and binds to positive cations (i.e. magnesium, calcium and bicarbonate) that are in high concentration in hard water (i.e. >200 ppm)
- Applying glyphosate during periods of high temperature and low humidity, resulting in the rapid loss of glyphosate in solution from leaf surfaces thereby reducing absorption
- Translocation of glyphosate in stressed plants can be reduced (Figure 3). Maximum glyphosate efficacy relies on translocation to the root and shoot tips. While this occurs readily in small seedlings, in larger plants, glyphosate is required to translocate further to the root and shoot tips to provide high levels of control
- Shading effects reducing leaf coverage resulting in sub-lethal effects
- As glyphosate strongly binds to soil particles. Application onto dust covered leaves can reduce efficacy
- Application factors such as speed and nozzle selection, boom height can reduce the amount of glyphosate coverage
- A combination of the above factors can reduce control thereby increasing the selection for resistance.
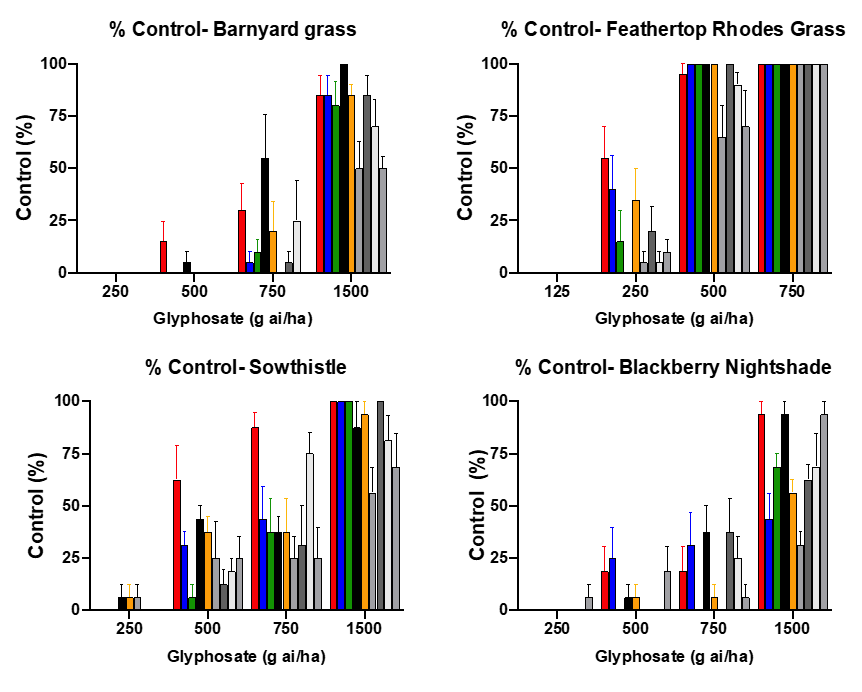
Figure 2. Control of 4 summer weed species with 9 different glyphosate formulations. (Plant Science Consulting).
Optimising glyphosate performance
The selection of glyphosate resistance can be minimised by considering the points above. A number of important pathways to improve glyphosate performance include:
Avoid applying glyphosate under hot dry conditions
Reduced control can occur if plants are water stressed. In an outdoor summer pot trial, the effect of water stress on glyphosate activity on barnyard grass was investigated. A sub-set of plants were water stressed 2 days prior to application with glyphosate. Plants at three growth stages were included. Control of stressed plants (drought treatment) was significantly less than of non-stressed plants at all growth stages (Figure 3).
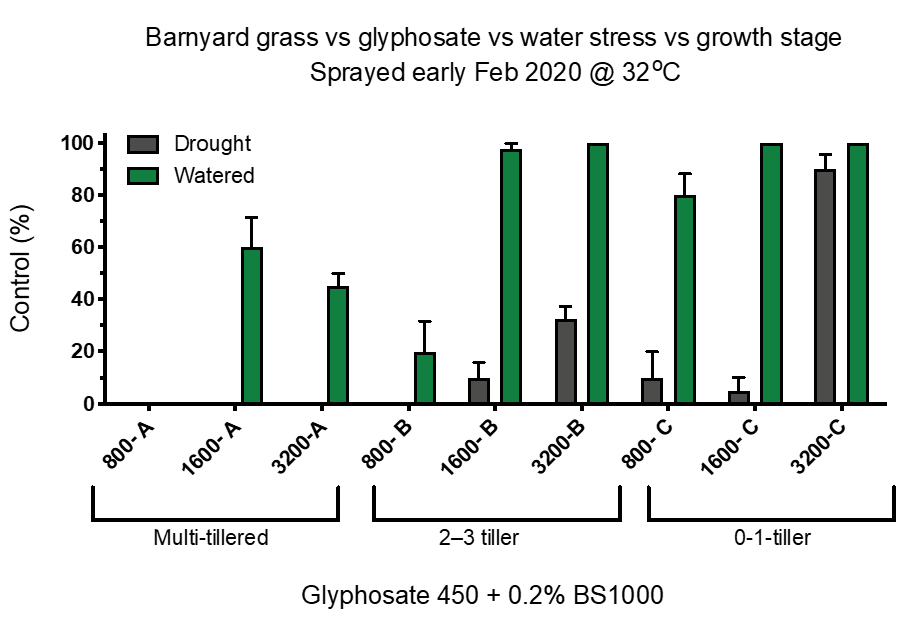
Figure 3. The effect of three rates of glyphosate on three growth stages of awnless barnyard grass, half not water-stressed and the other half water-stressed (Plant Science Consulting).
The effect of temperature on control of glyphosate resistant sowthistle from NSW was recently investigated. Initial trials have confirmed greater control with glyphosate at lower temperatures, particularly of resistant biotypes (Table 1). These findings suggest that applying glyphosate at lower temperatures can improve control of glyphosate resistant sowthistle. At lower temperatures glyphosate remains in liquid form on plant surfaces longer leading to greater uptake, particularly under higher humidity. This allows more hours on the leaf for glyphosate to penetrate. Maximising glyphosate uptake is therefore likely to improve weed control and factors such as lower temperature and higher humidity influence uptake. A pot trial investigating the effect of 20oC and 30oC on glyphosate efficacy identified a higher LD50 required at 30oC. This indicates that approximately 2.5x more glyphosate was required to control the resistant populations at 30oC, as opposed to the same populations grown at 20oC
Table 1. Effect of temperature in control of four biotypes of sowthistle from NSW with Glyphosate 540g/L. Data is LD50= dose required to kill 50% of the population.
Biotypes | Resistance level | LD50 (g a.i/ha) | |
|---|---|---|---|
20oC | 30oC | ||
Yellow | strong | 439 | 962 |
Crocket | strong | 389 | 919 |
White | weak | 132 | 389 |
GI | susceptible | 135 | 152 |
Improving water quality and glyphosate activity by using ammonium sulfate (AMS)
The addition of AMS has several functions. One is to soften water by combining to positively charged ions such as magnesium and calcium common in hard water. The negative charged sulfate ions combine with the positive cations preventing them from interacting with glyphosate and reducing glyphosate solubility and leaf penetration. Additionally, AMS has been shown to independently improve glyphosate performance, as the ammonium ions can work with glyphosate to assist cell entry, increasing uptake and activity. In a pot trial conducted with soft water, ammonium sulfate was shown to significantly improve control of ryegrass with 222 mL/ha (100 g ai/ha) of glyphosate 450 (Figure 4). As a general rule, growers using rainwater (soft) should consider 1% AMS, if using hardwater (i.e. bore, dam) 2% AMS is recommended. The addition of a wetter resulted in a further improvement of herbicide efficacy.
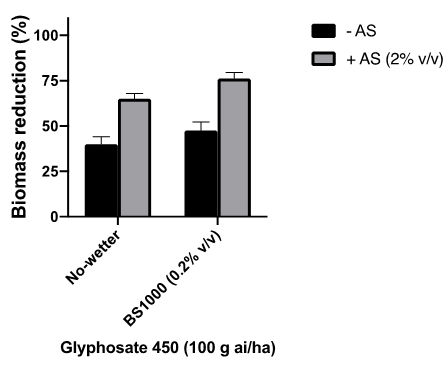
Figure 4. Effect of liquid ammonium sulfate and wetter on glyphosate for ryegrass control.
(A sub lethal rate was used to differentiate between treatment differences. Plants were grown and sprayed under optimum conditions).
Wild oat resistance
Over 120 samples of wild oats were received by Plant Science Consulting in 2020 from NSW and Qld, some as plant samples surviving a herbicide application (20 samples) during the cropping season and 100 seed samples currently being resistance tested. The most common mode of action where resistance has been identified to is Group A herbicides such as FOPs, tralkoxydim (eg. Achieve®) and pinoxaden (eg. Axial®). Increasing the rate of these herbicides to the top label rate seldomly improves control. From the plant samples tested over the last winter and spring, 65% were confirmed resistant to Group A herbicides and 35% not resistant, even though many survived a herbicide application in the field. A finding after testing hundreds of samples over the past decade is that clethodim controls wild oats resistant to most other Group A herbicides when used at the top label rate on young (3-leaf to early tillering) and non-stressed plants.
Random weed survey testing has been recently conducted in Qld (Table 2). In 34% of paddocks wild oats was confirmed resistant to clodinafop, with 3% of samples also resistant to Atlantis. A similar result was identified in NSW. This indicates that in a significant number of cases, poor wild oat control with FOP herbicides is due to herbicide resistance. A herbicide resistance test can determine if a poor paddock result is due to herbicide resistance. For more information see the website at the end of this document.
Table 2. Incidence of paddocks containing resistant wild oats as determined by GRDC funded random weed surveying and pot testing by Charles Sturt University, Waggga
Herbicide | QLD | NSW |
|---|---|---|
Clodinafop (eg. Topik) | 34 | 29 |
Clethodim (eg. Select) | 0 | 1 |
Mesosulfuron (Atlantis) | 3 | 4 |
Triallate (eg. Avadex Xtra_ | 0 | 0 |
Glyphosate | 0 | 0 |
Nr of samples | 64 | 511 |
Wild oat resistance to Group B herbicides is seldomly detected in resistance tests from Qld and in the case of resistant populations, the level of resistance is usually low. No resistance to triallate has been confirmed. Flamprop-methyl, previously registered as Mataven® and now available as several generic brands, is being included by some growers in their resistant test options.
Discrepancy between resistance testing and paddock failures
In some cases, plants that survived herbicides in the paddock are not resistant. Reasons for the discrepancy between the paddock and a resistance test can include poor application, antagonistic tank mixes, inferior herbicide quality, poor water quality, incorrect adjuvants, or a combination of the above. In addition, as wild oats are particularly prone to stressing by environmental extremes such as frost, heat and drought, herbicide failures can be due to these factors and not herbicide resistance. For this reason, it is of particular importance to have survivors tested for herbicide resistance, either by testing the seed or plants. Find more information here.
Summary
Since the first detection of glyphosate resistance in 1998 the number of resistant cases in several species has rapidly increased. Decades of strong selection pressure resulting from repeated use of glyphosate, coupled with application under suboptimum conditions and poor application has played a major role. Understanding the variables that affect glyphosate is important to optimise control. Resistance testing verifies that poor wild oat control in the field is not always due to herbicide resistance.
Acknowledgements
The information from Figure 1 is from research conducted at the University of Adelaide.
References
Powles SB, Lorraine-Colwill DF, Dellow JJ, Preston C. 1998. Evolved resistance to glyphosate in rigid ryegrass (Lolium rigidum) in Australia. Weed Science. 46:604–607
Contact details
Peter Boutsalis
Plant Science Consulting P/L
University of Adelaide, Waite Campus, Glen Osmond SA 5064
Herbicide resistance testing website: www.plantscienceconsulting.com.au
@PBoutsalis
Email: peter.boutsalis@adelaide.edu.au
Email: info@plantscienceconsulting.com.au
® Registered trademark
