Annual ryegrass weed management and paraquat resistance
Annual ryegrass weed management and paraquat resistance
Author: Peter Boutsalis (School of Agriculture, Food & Wine), (Plant Science Consulting) Ben Fleet, Gurjeet Gill, Christopher Preston (School of Agriculture, Food & Wine, University of Adelaide) | Date: 10 Feb 2022
Take home messages
- According to a recent national weed survey, resistance to pre-emergence herbicides in annual ryegrass is low.
- Paraquat resistance in broadacre paddocks has been confirmed.
- Monitoring for resistance using herbicide resistance testing is important to protect against glyphosate and paraquat resistance increasing.
- Glyphosate should be used strategically, even if glyphosate resistance is present to protect against paraquat resistance.
National herbicide resistance weed survey 2020-2023
A national weed survey commenced in 2020 through GRDC investment. In a national collaboration between universities, over 1500 paddocks were sampled across WA, SA, Vic, Tas, NSW and Qld in 2020 and 2021. Grower paddock details were supplied by agronomists and each university randomly selected a set number of paddocks in their respective state. After sampling, all the annual ryegrass were sent to the University of Adelaide for testing, barley grass, brome and wild radish to the Australian Herbicide Resistance Initiative (AHRI), wild oats and sowthistle to Charles Sturt University (CSU). Using this approach, the national collection of each species will be tested together. In 2021, the national ryegrass collection was tested with pre-emergence herbicides with the post-emergence testing to be conducted in 2022 (Tables 1 and 2).
Table 1: Percent of paddocks detected with resistant ryegrass treated with the recommended label rate of pre-emergence herbicides. Resistance is defined as a sample where ³20% plant survival was detected in the 2021 pot trials.
State | Herbicides | |||||
|---|---|---|---|---|---|---|
Trifluralin | Boxer Gold® | Sakura® | Propyzamide | Luximax® | Overwatch™ | |
National | 12 | 2 | 0 | 0 | 0 | 0 |
SA | 38 | 1 | 0 | 0 | 0 | 0 |
VIC | 21 | 9 | 0 | 0 | 0 | 0 |
NSW | 0 | 1 | 0 | 0 | 0 | 0 |
WA | 4 | 2 | 0 | 0 | 0 | 0 |
TAS | 0 | 0 | 0 | 0 | 0 | 0 |
Table 2: Number of paddocks where ryegrass was collected and tested from each state.
State | Herbicides | |||||
|---|---|---|---|---|---|---|
Trifluralin | Boxer Gold | Sakura | Propyzamide | Luximax | Overwatch | |
National | 1353 | 1202 | 1202 | 1202 | 1202 | 1202 |
SA | 279 | 266 | 266 | 266 | 266 | 266 |
VIC | 183 | 179 | 179 | 179 | 179 | 179 |
NSW | 317 | 273 | 273 | 273 | 273 | 273 |
WA | 554 | 465 | 465 | 465 | 465 | 465 |
TAS | 20 | 19 | 19 | 19 | 19 | 19 |
The trends in resistance to pre-emergence herbicides in ryegrass supports the findings from previous surveys. The greatest incidence of resistance to trifluralin was detected in SA (38%) followed by Victoria (21%), WA (4%) and 0% in NSW and Tasmania. The only other resistance detected was to Boxer Gold, the highest (9%) in Victoria. No resistance to field rates of Sakura, Propyzamide, Luximax and Overwatch was detected. These results suggest several herbicide options for the pre-emergence control of ryegrass remain.
Improving ryegrass weed control
Resistance levels within individuals in a population can vary and, in many cases, a resistant plant can be killed with a robust field rate under optimum spray and growth conditions. This is most common in plants with weak resistance mechanisms, particularly at early growth stages, with herbicides such as clethodim and glyphosate. Young plants possess thinner cuticles making herbicide entry easier. However, plants with strong resistance mechanisms, such as certain Group 1 (A) and 2 (B) target site resistance, are difficult to control, even at young growth stages. The high frequency of Group 2 (B) target site resistance in certain weed species such as annual ryegrass, explains why this species is sometimes difficult to control with these herbicides. Fortunately, control with alternative diverse mode of action pre-emergence herbicides is possible.
Optimising paraquat performance
In order to maximise the efficacy of paraquat consider the below:
- Use high quality paraquat products and surfactants where recommended.
- Since paraquat does not translocate, coverage is more important than for glyphosate. Use nozzles that will ensure uniform coverage at water rates of at least 100L/ha on small seedlings and higher water rates on more advanced growth stages.
- Avoid combining paraquat with too many other active ingredients to reduce the likelihood of antagonism, particularly with low water volumes.
- Avoid using muddy water. (see useful resources at end of paper).
- Avoid applying paraquat during periods of high light and temperature and low humidity, to avoid rapid activation and loss from leaf surfaces, particularly if targeting tillering plants. Application in low light conditions will improve activity.
- Consider using higher label rates if there is considerable shading.
- Maximise application by adhering to lower speeds and using the correct nozzles, pressure and boom height.
Glyphosate and paraquat resistance
Across southern Australia, the most important species developing glyphosate resistance is annual ryegrass. It is very important to test for glyphosate resistance to ensure the correct weed control strategies are implemented. Even if glyphosate resistance is confirmed it can still be used strategically. Unlike resistance to some Group 1 (A) and Group 2 (B) herbicides where the level of resistance in an individual can be high, glyphosate resistance often begins with weak resistance in a low number of plants and if left uncontrolled can increase over time, particularly for cross-pollinating species such as ryegrass.
If a resistance test was conducted and it confirmed a high survival rate (for example, 100% of plants tested resistant), don’t panic. If the sampling for resistance was comprised of very few individuals identified in the paddock after the glyphosate application, whether plants (Quick-Test) or seeds (Seed Test) were tested, then the true incidence of resistance is very low. Management in the subsequent season should actively target to control any survivors. That doesn’t necessarily imply not to use glyphosate. Over relying solely on paraquat as the only knockdown can impose strong selection pressure for the development of resistance. A double knock approach involving glyphosate (to control the majority of susceptible individuals) followed with a robust rate of paraquat 1—5 days later is ideal (Figure 1). This approach reduces the selection pressure because the number of individuals exposed to paraquat is reduced since glyphosate does most of the ‘heavy lifting’. Tank-mixing glyphosate and paraquat is not recommended to avoid strong antagonism. Paraquat resistance was confirmed in the 2017 South-East SA random weed survey in 7% of ryegrass samples. In lucerne and white clover seed crops paraquat alone is commonly used to control ryegrass, as these crops can regenerate from the paraquat burndown. It is, therefore, not surprising that the initial paraquat resistant cases were identified in lucerne and white clover paddocks. A low number of paraquat resistant ryegrass cases have, recently been confirmed in cropping paddocks in South-Western Victoria and South-Eastern SA. Some of these biotypes are also resistant to glyphosate. One of the paraquat resistant sites had been exposed to less than five applications of paraquat, suggesting that the resistance had been brought into the paddock from an outside source. Monitoring for survivors after paraquat use is necessary, with testing recommended to prevent paraquat resistance increasing.
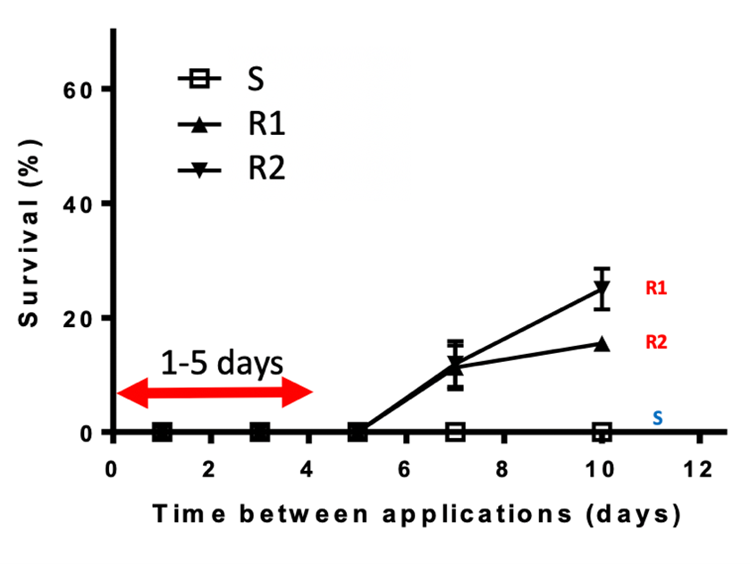
Figure 1. Double knock timing. Glyphosate applied onto a susceptible (S) and two glyphosate resistant ryegrass biotypes (R1 & R2) followed by paraquat 1, 3, 5, 7 and 10 DAA. Trial work conducted by Dr Christopher Preston, The University of Adelaide.
The use of an effective pre-emergent herbicide or combination is recommended to control any subsequent germination. With delayed germination becoming more prevalent in some ryegrass biotypes, a resistance test would aid in the identification of whether there were effective post-emergent herbicide options available to control potential glyphosate resistance. It is not advisable to grow a GM canola crop unless clethodim/butroxydim is effective, so clethodim/butroxydim (or clethodim + glyphosate) can be used to control ryegrass and glyphosate to control susceptible ryegrass and other target species. The use of atrazine is an important tool as it controls many weed species including ryegrass, with the added benefit of only few cases of resistance detected. Tank-mix combinations of atrazine and clethodim have recently been shown to be antagonistic in controlling ryegrass, therefore separating these two products is recommended to maintain control with either product (Table 3). Clethodim with no atrazine provided 10% greater control than Clethodim + Atrazine (tank-mix). Control improved the longer the separation between the first and second treatment.
Table 3: Increase in control (%) of treatments compared to a Clethodim + Atrazine tankmix on 3-leaf ryegrass in outdoor pot trials. Data is the pooled response of a susceptible and 3 DIM-resistant ryegrass biotypes. The trial was repeated with Atrazine applied followed by the Clethodim treatments and vice versa (second trial). Rates are (1) Clethodim EC240 @ 500mL/ha and Atrazine WG900 @ 2kg/ha. Hasten and liquid AMS at 1% were included with each treatment. The Clethodim + Atrazine (tank-mix) treatment response was converted to 0 to calculate the improvement in control of the other treatments.
Treatments | Time after first herbicide | % Increase in control over Clethodim + Atrazine tankmix |
|---|---|---|
Clethodim + Atrazine (tank-mix) | 0 | 0 |
Clethodim only | no atrazine | 10 |
Clethodim-Atrazine sequence | 10 min | 11 |
Clethodim-Atrazine sequence | 2 days | 17 |
Clethodim-Atrazine sequence | 7 days | 30 |
Crop topping with glyphosate where glyphosate resistance has been confirmed is not advisable as it may serve to sterilise susceptible ryegrass seed and leave resistant plants behind to preferentially cross pollinate and fast-track glyphosate resistance (Figure 2). A seed-sterilisation field trial was conducted in 2016 at a site with confirmed glyphosate resistance. Viability testing of the seed after maturation revealed that the reduction in seed germination was between 9-22%, indicating that at least 80% of the seed remained viable. Glyphosate was therefore not effective in sterilising glyphosate resistant ryegrass.
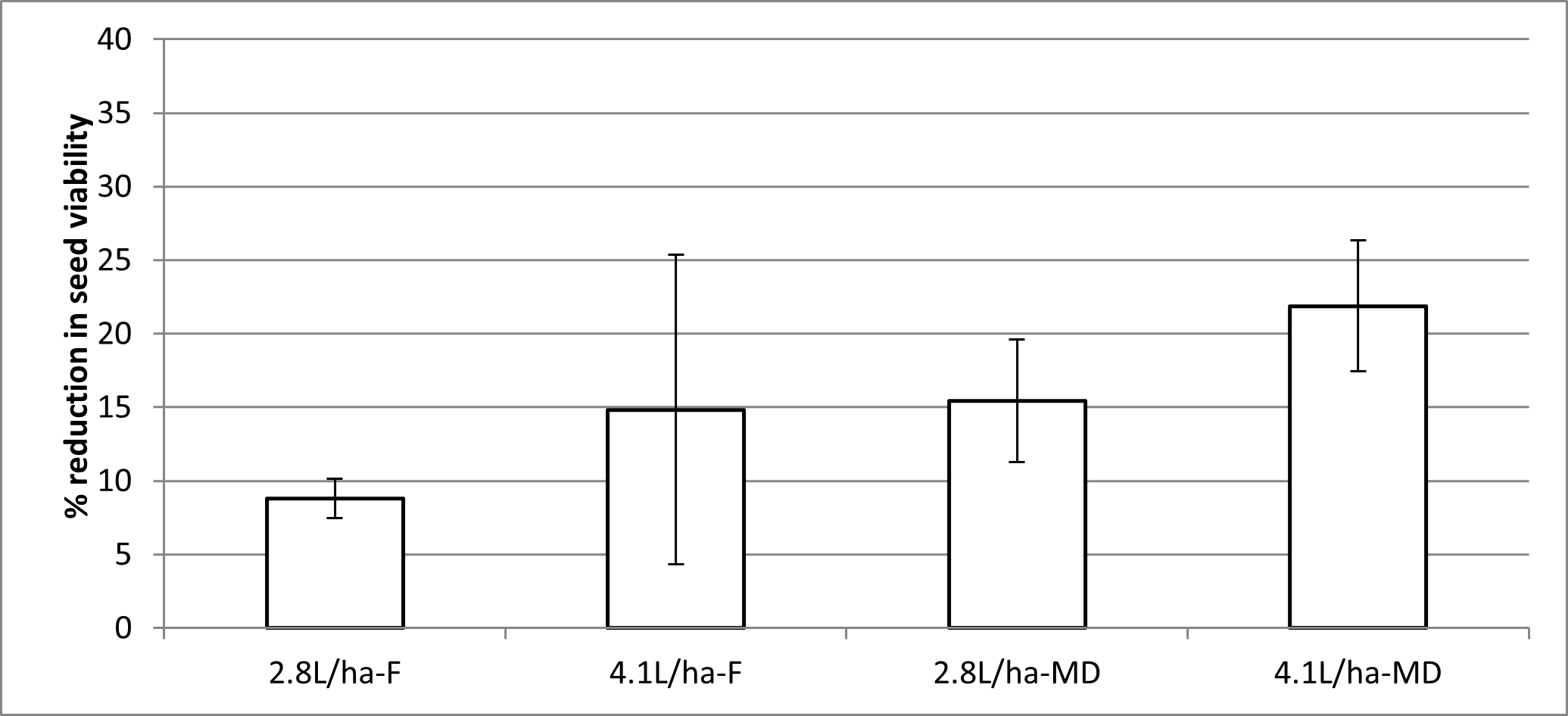
Figure 2. Reduction in viability of ryegrass seed after crop-topping with Weedmaster DST at two timings, F - flowering and MD = milky dough. Trial conducted at Roseworthy SA in 2016.
Crop rotation with pulses is an option. There are several robust herbicide options available for combatting glyphosate resistance in a pulse crop such as propyzamide, carbetamide, higher Group 1 (DIM) registered rates and crop-topping with paraquat.
Conclusion
Several pre-emergent herbicide options remain to control multiple-resistant ryegrass as indicated by recent national weed surveys. There are several factors that can contribute to poor weed control with resistance being only one of them. Optimising application equipment, timing and understanding environmental factors that reduce herbicide efficacy is important. Glyphosate and paraquat can be used strategically, even if resistance is present.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both research cooperation and the support of the GRDC, the author would like to thank them for their continued support. The information for the random weed surveys was undertaken as part of GRDC project UCS1306-001RMX and UCS2008-001RTX.
Useful resources
Understanding Post Emergent Herbicide Weed Control
Contact details
Peter Boutsalis
Plant Science Consulting P/L
University of Adelaide
Waite Campus, Glen Osmond SA 5064
peter.boutsalis@adelaide.edu.au
Plant Science Consulting
@PBoutsalis
GRDC Project Code: UCS1306-001RMX, UCS2008-001RTX,
