Can we improve the roots of durum wheat to maximise yield?
Author: Lee Hickey (University of Queensland), Anton Wasson (CSRIO), Yichen Kang (University of Queensland), Samir Alahmad (University of Queensland) | Date: 24 Feb 2022
Take home message
- A major gene (QTL) for seminal root angle (qSRA-6A) influences root distribution and could be used by durum breeders to develop new cultivars with optimal root systems
- Introgression lines were generated by backcrossing the major QTL into an elite durum cultivar
- Extensive root coring of the introgression lines under field conditions identified lines with significantly altered root distributions
- Durum lines carrying the wide allele for the QTL tend to produce more roots in the upper soil layer, which can enhance water extraction from the upper soil layer. This type of root system can enhance yield potential in environments where soil moisture is not limiting
- The introgression lines provide valuable genetic resources for plant breeders and researchers to further study the value of root systems in different production scenarios.
Introduction
Root system architecture is a representation of the spatial and temporal distribution of root growth in the soil and thus is critical for water uptake throughout the season. Root systems are complex in nature and comprise a large number of component traits, many of which are important, but their yield advantage is yet to be thoroughly explored (Ober et al., 2021). For durum wheat, a trait that may influence the direction and distribution of roots in the soil space is seminal root angle. Seminal root angle determines the direction of root growth and distribution of roots in different layers of the soil profile. For instance, a narrow root angle could lead to deeper root growth which may be beneficial for accessing moisture in deep soil layers under terminal drought conditions. On the other hand, a wide root angle could lead to a higher proportion of shallow root growth, which may be beneficial for taking up nutrients and soil moisture in the upper soil layers. The value of different root systems is highly context-dependent and likely changes depending on the environmental conditions and management practices.
Thus far, a substantial body of research suggests that optimised root systems can improve resource acquisition, resulting in improved crop yield under both favourable and adverse conditions. Modern crop improvement programs, however, struggle to incorporate selection for root traits, largely due to the challenges associated with phenotyping root structure, a lack of understanding of the genetic controls of root system architecture, and an inability to assess the value of root traits in specific environment types.
In this study, we investigated the ability to modify root system architecture by targeting selection of the seminal root angle trait. This was achieved by backcrossing a major QTL for seminal root angle (qSRA-6A) into the durum cultivar DBA Aurora. The changes to RSA were explored through a series of phenotyping experiments performed under controlled and field conditions. We anticipate these results will help guide the development of new cultivars with optimised root systems that maximise yield.
Investigating the potential to manipulate root systems of elite durum cultivars
The major gene (i.e., QTL) modulating seminal root angle, named qSRA-6A (Alahmad et al., 2019), was introgressed into the durum wheat cultivar DBA Aurora . This was achieved by applying marker-assisted selection using linked DNA markers in a backcrossing scheme under speed breeding at The University of Queensland, QLD, Australia. This resulted in the development of BC2F5 introgression lines that were 87.5% genetically similar to DBA Aurora but differed for qSRA-6A. A total of 11 introgression lines were developed, including 6 which carried the ‘narrow’ allele and 5 which carried the ‘wide’ allele.
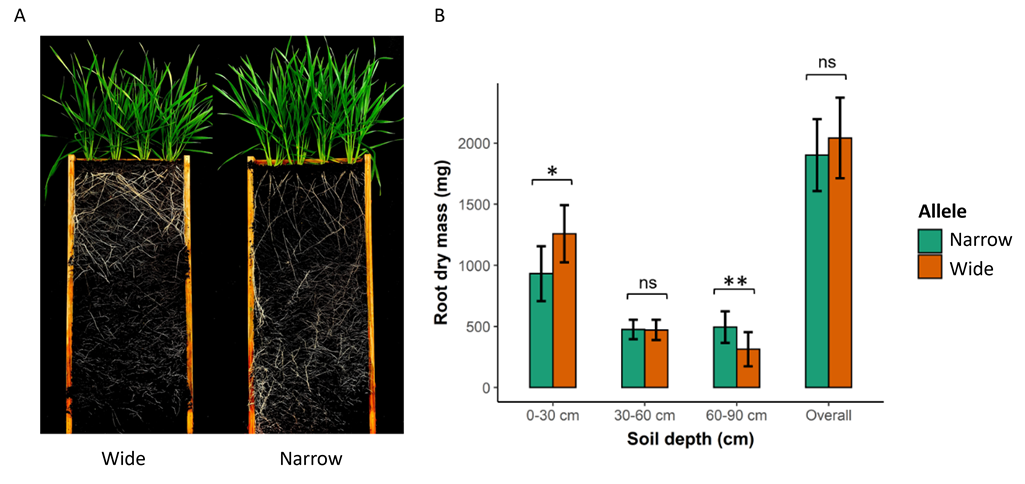
To determine whether introgression of qSRA-6A into the DBA Aurora background was successful, the 11 introgression lines and DBA Aurora were evaluated in a rhizobox phenotyping platform under controlled glasshouse conditions (St Lucia campus, The University of Queensland, QLD, Australia). The 12 genotypes were evaluated using a randomised complete block design with four replicates per genotype. Rhizoboxes were constructed from wood panelling and lined with black plastic to contain the potting mix and to facilitate root imaging. The rhizoboxes were filled with UQ23 potting media (70% composted pine bark 0–5 mm, 30% cocoa peat, mineral fertiliser) pre-mixed with 2g/L of Osmocote® slow release fertiliser and a total of four plants were grown in each. After 44 days of growth, the rhizoboxes were opened and roots extracted from three depths: upper (0-30cm), middle (30-60cm) and bottom (60-90cm) layers. The roots were washed, dried and weighed to determine dry root mass (mg) in each layer.
Unpaired t-tests revealed that introgression lines carrying the ‘wide’ allele for the QTL produced significantly more roots in the upper layer (P ≤ 0.05), whereas introgression lines carrying the ‘narrow’ allele produced significantly more roots (P ≤ 0.01) in the deepest layer (Figure 1A, B). Notably, no significant difference was observed in the total (overall) root biomass produced by ‘wide’ and ‘narrow’ lines (Figure 1B). Thus, targeted selection of a major QTL for root angle successfully changed the distribution of roots in the DBA Aurora background without impacting the total carbohydrate allocated to root growth.
Characterising the elite pre-breeding lines for root distribution in the field
To explore root distribution changes under field conditions, the DBA Aurora introgression lines were evaluated in a field trial conducted at the University of Queensland’s Gatton Research Farm (27°32'34"S; 152°19'59"E) in 2021. The 12 genotypes were sown in standard 7-row plots (6m long x 1.52m wide) according to a randomised complete block design with eight replicates, where four replicates were mulched at flowering for root coring (Figure 2A) and four replicates were machine harvested at maturity to measure grain yield.
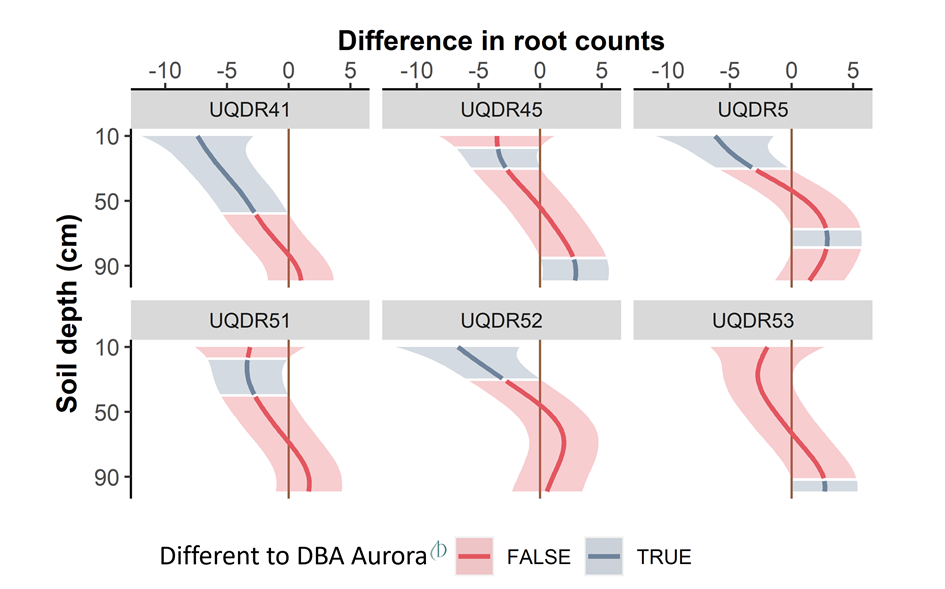
Root phenotyping was performed using a ‘core-break’ method described by Wasson et al. (2014). This involved careful removal of intact soil cores, breaking the core into 10cm intervals (from 10-100cm), and visual counting of the number of roots visible on each surface of the break (Figure 2A:C). The number of root breaks per interval down the soil profile correlates well with root length density (Wasson et al., 2014). To account for within-plot variability, four cores were sampled per plot from the two inner and intra rows of the ‘destructive’ plots. Raw root count data were modelled to account for variations between operators and spatial effects across the trial. The root distribution for each genotype was estimated using a smoothing function with a generalized additive model, which had the effect of integrating over all the depths in the profile.

A total of 6 of the 11 introgression lines showed root distribution patterns that were significantly different to DBA Aurora (Figure 3). Five of the six lines produced significantly fewer roots in the upper soil layers and two of these lines, UQDR045, a ‘narrow’ line, and UQDR5, a ‘wide’ line, also produced a significantly higher proportion of roots in the deeper soil layer. ‘Narrow’ line (UQDR53) showed a root distribution that was similar to DBA Aurora with the exception of more roots in the deepest soil layer.
The introgression lines generated in this study show significant difference in root distribution under field conditions, thus providing valuable genetic resources for plant breeders and researchers to further study the value of different root systems in a range of production scenarios.
Understanding the relationship between root distribution, water extraction and durum yield
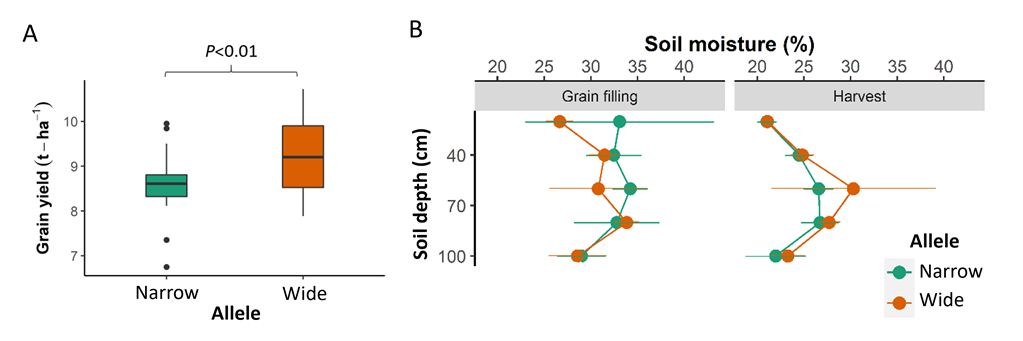
Interestingly, the introgression lines carrying the ‘wide’ allele produced significantly higher yield compared to lines carrying the ‘narrow’ allele in the Gatton 2021 trial (Figure 4A).
To investigate the relationship between root distribution, water extraction and yield in this environment, soil coring was performed during early grain filling and harvest for a subset of the introgression lines. Coring was performed using a hydraulic soil corer mounted to the back of a trailer. A total of three intact cores were sampled per plot. The cores were divided into 20cm intervals down to 1m depth. Soil samples were weighed and dried to calculate soil moisture. Mean soil moisture at each depth were compared for groups of lines carrying the ‘narrow’ and ‘wide’ alleles using an unpaired t-test.
Analysis of the soil moisture data at early grain filling revealed a trend that lines carrying the ‘wide’ allele tended to extract more soil moisture in the upper soil layers in comparison with the lines carrying the ‘narrow’ allele, particularly within the top 20cm depth (p = 0.065). At harvest, the soil moisture under ‘narrow’ and ‘wide’ lines was similar at all soil depths (Figure 4B). This suggests the timing of water extraction was critical, where higher water extraction early in the season was likely a key contributor to the higher yield produced by the lines carrying the ‘wide’ allele. Thus, lines with a wider and shallower root system were able to extract more water during the vegetative stage and take advantage of in-season rainfall, which was likely converted to biomass and subsequently yield.
Notably, the yield trial was conducted on a deep cracking clay soil with a high water-holding capacity, and at the time of sowing the soil had a full profile of water. The trial also received 326mm of rainfall during the season (May to November) with 177mm rainfall from flowering to harvest time which meant water supply was likely not limiting during the grain-filling period. It is important to consider if the Gatton trial received less in-season rainfall, the lines carrying the ‘narrow’ allele may have produced a yield advantage due to water savings early in the season.
Overall, these results highlight the value of designing cultivars that are capable of producing roots where soil resources are available. In this environment, root proliferation in the upper soil layers was advantageous due to water and nutrient availability. Not only was water available but also nutrients including nitrogen and phosphorus that are critical to support crop growth and performance. However, the yield outcome would likely be different in other environments where water and nutrient resources vary either temporally or spatially.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support. We also give thanks to Charlotte Rambla and Sarah Van Der Meer for assistance with root phenotyping, and CSIRO staff for maintaining the field trial at Gatton.
References
Alahmad S, El Hassouni K, Bassi FM, Dinglasan E, Youssef C, Quarry G, Aksoy A, Mazzucotelli E, Juhász A, Able JA, Christopher J, Voss-Fels KP, Hickey LT (2019) A major root architecture QTL responding to water limitation in durum wheat. Frontiers in Plant Science 10:10-436
Ober ES, Alahmad S, Cockram J, Forestan C, Hickey LT, Kant J, Maccaferri M, Marr E, Milner M, Pinto F, Rambla C. (2021) Wheat root systems as a breeding target for climate resilience. Theoretical and Applied Genetics 26:1–8
Wasson AP, Rebetzke GJ, Kirkegaard JA, Christopher J, Richards RA, Watt M (2014) Soil coring at multiple field environments can directly quantify variation in deep root traits to select wheat genotypes for breeding. Journal of Experimental Botany 65:6231–49
Contact details
Associate Professor Lee Hickey
Centre for Crop Science, Queensland Alliance for Agriculture and Food Innovation
The University of Queensland
Brisbane Qld 4072 Australia
Ph: 07 3365 4805
Email: l.hickey@uq.edu.au
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
GRDC Project Code: UOQ1903-007RTX,
Was this page helpful?
YOUR FEEDBACK
