Fungicide resistance update - national situation and issues for the northern grains region
Take home messages
- Monitoring and analysis of pathogen populations by CCDM in 2021 revealed new resistance mutations affecting fungicide performance for the first time in Australia and in other cases existing mutations being more widespread and affecting more states
- In a field trial in NE Victoria which combined field efficacy with laboratory analysis, testing has revealed significant differences in DMI (Group 3, triazole) performance for control of wheat powdery mildew (WPM)
- The results illustrated that the weaker compounds (triadimefon, epoxiconazole, tebuconazole, cyproconazole and propiconazole) provided less than 50% control of WPM
- Fungicide resistance and reduced sensitivity can be slowed down by using integrated disease management (IDM) approaches that reduce the number of fungicide applications required
- To ‘slow the train that’s heading to fungicide resistance’, growers and advisers need to adopt fungicide resistance management strategies that avoid repeated applications of the same modes of action and active ingredients
- IDM strategies can include crop rotation, stubble management, green bridge control, sowing more disease resistant (avoid susceptible) cultivars, nutrition and canopy management (e.g. grazing) to minimise disease pressure.
Background
Fungicide resistance is a major concern for Australian growers as it potentially reduces the efficacy of fungicides and their ability to protect grain yield and profit potential. To minimise the yield gap on cropping farms, it is essential to maintain impact of these agrichemicals through fungicide resistance management strategies.
The first step in recognising the significance of this problem is to understand which pathogens are developing issues and to which fungicide actives.
The research reported in this paper includes fungicides that may not be registered in Australia either alone or in combination with other actives for the diseases mentioned. Their use was necessary for the express purpose of determining the resistance profile for specific mode of action groups and actives. Only products that are registered for use in Australia should be used and in accordance with directions for use on their respective labels.
What is the current status of fungicide resistance and reduced sensitivity in Australia?
Over the last decade the Fungicide Resistance Group (FRG) at the Centre for Crop and Disease Management, (CCDM at Curtin University) has been working with industry and other researchers to establish a fast and cost-effective monitoring system for fungicide resistance of common fungal pathogens of broad acre grain crops. Current cases of fungicide resistance and reduced sensitivity in Australian broadacre grain crops are outlined in Table 1.
Table 1. Fungicide resistance and reduced sensitivity cases identified in Australian broadacre grains crops.
Disease | Pathogen | Fungicide Group | Compounds affected | Region (status*) | Industry implications |
|---|---|---|---|---|---|
Barley powdery mildew | Blumeria graminis f.sp. hordei | 3 (DMI) | Tebuconazole | WA (R), Qld, NSW, Vic, Tas, (L) | Field resistance and reduced sensitivity to some actives |
Wheat powdery mildew | Blumeria graminis f.sp. tritici | 3 (DMI) | Propiconazole | NSW, Vic (R), Tas, SA (L) | Field resistance to some actives in NSW and Vic. The gateway mutation is the first step towards resistance. This mutation does not seem to reduce efficacy in the field but combined with other mutations can affect DMI efficacy |
11 (QoI) | Azoxystrobin | Vic, Tas, SA & NSW (R) | Field resistance to all Group 11 fungicides | ||
Barley net-form of net blotch | Pyrenophora teres f.sp. teres | 3 (DMI) | Tebuconazole Epoxiconazole | WA (R), | Field resistance and reduced sensitivity to some actives |
7 (SDHI) | Fluxapyroxad | SA (R & RS), VIC (L) | Reduced sensitivity or resistance depending on the frequency of resistant population | ||
Barley spot-form of net blotch | Pyrenophora teres f.sp. maculata | 3 (DMI) | Tebuconazole Epoxiconazole | WA (R, RS) | Field resistance to some actives |
7 (SDHI) | Fluxapyroxad | WA (R, RS) | Field resistance and reduced sensitivity | ||
Wheat septoria | Zymoseptoria tritici | 3 (DMI) | Tebuconazole | NSW, Vic, SA, Tas (RS) | Reduced sensitivity |
11 (QoI) | Azoxystrobin | SA, (Millicent region) (R) | Frequency of A143 mutation in Millicent region unknown. 32 STB samples collected from 29 locations across Victoria, South Australia and NSW in 2021 did not detect the mutation associated with resistance to QoI fungicides | ||
Canola Blackleg disease | Leptosphaeria maculans | 3 (DMI) | Tebuconazole Flutriafol | VIC, NSW, SA, WA (RS) | Reduced sensitivity |
*Table 1 definitions:
Reduced sensitivity (RS): Fungi are considered as having reduced sensitivity to a fungicide when a fungicide application does not work optimally but does not completely fail. In most cases, this would be related to small reductions in product performance which may not be noticeable at the field level. In some cases, growers may find that they need to use increased rates of the fungicide to obtain the previous level of control. Reduced sensitivity needs to be confirmed through specialised laboratory testing. Note that mutations that cause field failure (full resistance) present at lower frequencies in a pathogen population would give similar field symptoms to mutations that cause small reductions in field performance but which do not cause field failure.
Resistant (R): Resistance occurs when the fungicide fails to provide an acceptable level of control of the target pathogen in the field at full label rates. Resistance needs to be confirmed with laboratory testing and be clearly linked with an unacceptable loss of disease control when using the fungicide in the field at full label rates.
Laboratory detection (L): Measurable differences in sensitivity of the pathogen to the fungicide when tested in the laboratory. Detection of resistance in the lab can often be made before the fungicide’s performance is impacted in the field.
Fungicide reduced sensitivity and resistance in NSW/SA/Victoria in 2021
The following section carries results from three states. Although resistance results from Vic and SA may seem less relevant to the northern GRDC region, they give us an early warning of potential issues in southern NSW where farming systems are more similar to SA and Victoria.
Wheat powdery mildew in the northern grains region
Wheat powdery mildew (WPM) was particularly problematic in NSW in 2020 but was less damaging in 2021.
Steven Simpfendorfer (NSW DPI) co-ordinated 22 samples of WPM for testing with CCDM over the last two seasons and the results revealed widespread fungicide reduced sensitivity in the DMIs and resistance in the QoIs (Table 2). The F136 mutation in WPM is a gateway mutation that doesn’t confer field resistance but in combinations with other mutations (which are still being characterised) in the same gene does confer reduced sensitivity in the field.
Table 2. Location of 22 wheat powdery mildew samples; 19 collected across NSW in 2020 and 3 in 2021 along with frequency of DMI (triazole) gateway and Qol (strobilurin) mutations.
Location | Year | Region | Variety | DMI F136 | Qol A143 |
|---|---|---|---|---|---|
Katamatite | 2020 | NE Vic | Scepter | 100% | 90% |
Katamatite | 2020 | NE Vic | Scepter | 100% | 90% |
Cobram | 2020 | NE Vic | Scepter | 100% | 46% |
Cobram | 2020 | NE Vic | Scepter | 100% | 28% |
Balldale | 2020 | SE NSW | Scepter | 100% | 98% |
Walbundrie | 2020 | SE NSW | Scepter | 100% | 5% |
Rennie | 2020 | SE NSW | Suntop | 85% | 27% |
Rennie | 2020 | SE NSW | Scepter | 85% | 20% |
Jerilderie | 2020 | SE NSW | Scepter | 100% | 37% |
Corowa | 2021 | SE NSW | Scepter | 100% | 94% |
Deniliquin | 2020 | SW NSW | Scepter | 99% | 35% |
Deniliquin | 2020 | SW NSW | Scepter | 99% | 20% |
Deniliquin | 2020 | SW NSW | Scepter | 83% | 20% |
Hillston | 2020 | SW NSW | Vittaroi | 96% | 21% |
Hillston | 2020 | SW NSW | Vixen | 94% | 3% |
Hillston | 2020 | SW NSW | Vixen | 85% | 6% |
Yenda | 2020 | SW NSW | Cobra | 100% | 44% |
Yenda | 2020 | SW NSW | Vixen | 100% | 12% |
Finley | 2021 | SW NSW | Scepter | 100% | 38% |
Edgeroi | 2020 | NE NSW | Lillaroi | 82% | 29% |
Wee Waa | 2020 | NW NSW | Bindaroi | 62% | 51% |
Wee Waa | 2021 | NW NSW | Aurora | 100% | 20% |
FAR working in collaboration with CCDM and NSW DPI ran an irrigated trial at Katamatite in NE Victoria in 2021 to determine the field performance of different modes of action and DMI active ingredients for control of WPM. The results illustrated some interesting differences in field performance which, whilst not all statistically significant, illustrated that the weaker compounds of triadimefon, epoxiconazole (Opus), tebuconazole, cyproconazole plus propiconazole (Bumper) were giving less than 50% control (Figure 1). Isolates from this trial were taken in October (post application) and the samples sent to CCDM for fungicide resistance testing. Analysis for the presence of the A143 mutation that affects WPM control globally when using group 11 QoIs (strobilurins) was present in all treatments (Figure 2) but as might be expected was highest in the experimental treatment that received straight strobilurin alone (azoxystobin - Mirador®). Therefore, although the WPM control within this experimental treatment was not the poorest (still less than 50% control) it indicates that the population that remains post application will be less effectively controlled. Clearly, we don’t apply this fungicide alone in Australia but in mixtures with DMIs, however it demonstrates the selection pressure that can occur in a season when we use fungicide actives that are at higher risk of resistance development in the pathogen. Significant differences to the untreated in the level of the QoI mutation in plots treated with DMIs and the Group 5 fungicide Prosper® (spiroxamine) will be investigated further.
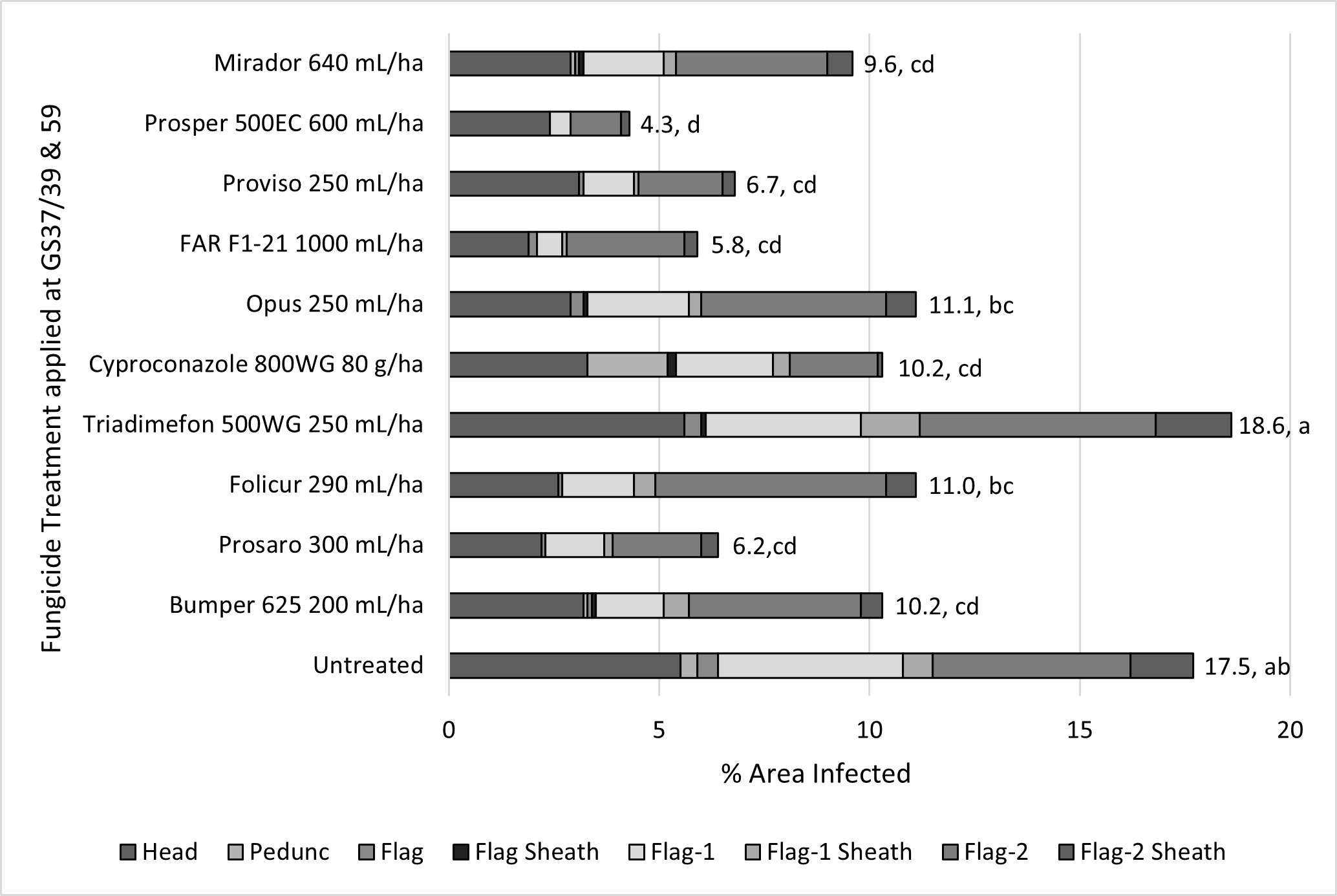 Figure 1. Influence of two spray fungicide application (GS37/39 and GS59) on wheat powdery mildew (WPM) infection on different components of upper canopy – cv Scepter, Katamatite, Vic 2021.
Figure 1. Influence of two spray fungicide application (GS37/39 and GS59) on wheat powdery mildew (WPM) infection on different components of upper canopy – cv Scepter, Katamatite, Vic 2021.
Notes: Data labels and statistical significance based on total WPM infection of all plant components listed
Notes: Please be aware that cyproconazole, FAR F1-21, Prosper and Mirador have been included in this experimentation as experimental treatments that currently cannot be used commercially in this form. These treatments were included to test the full range of available individual fungicide actives some of which are only approved in mixtures
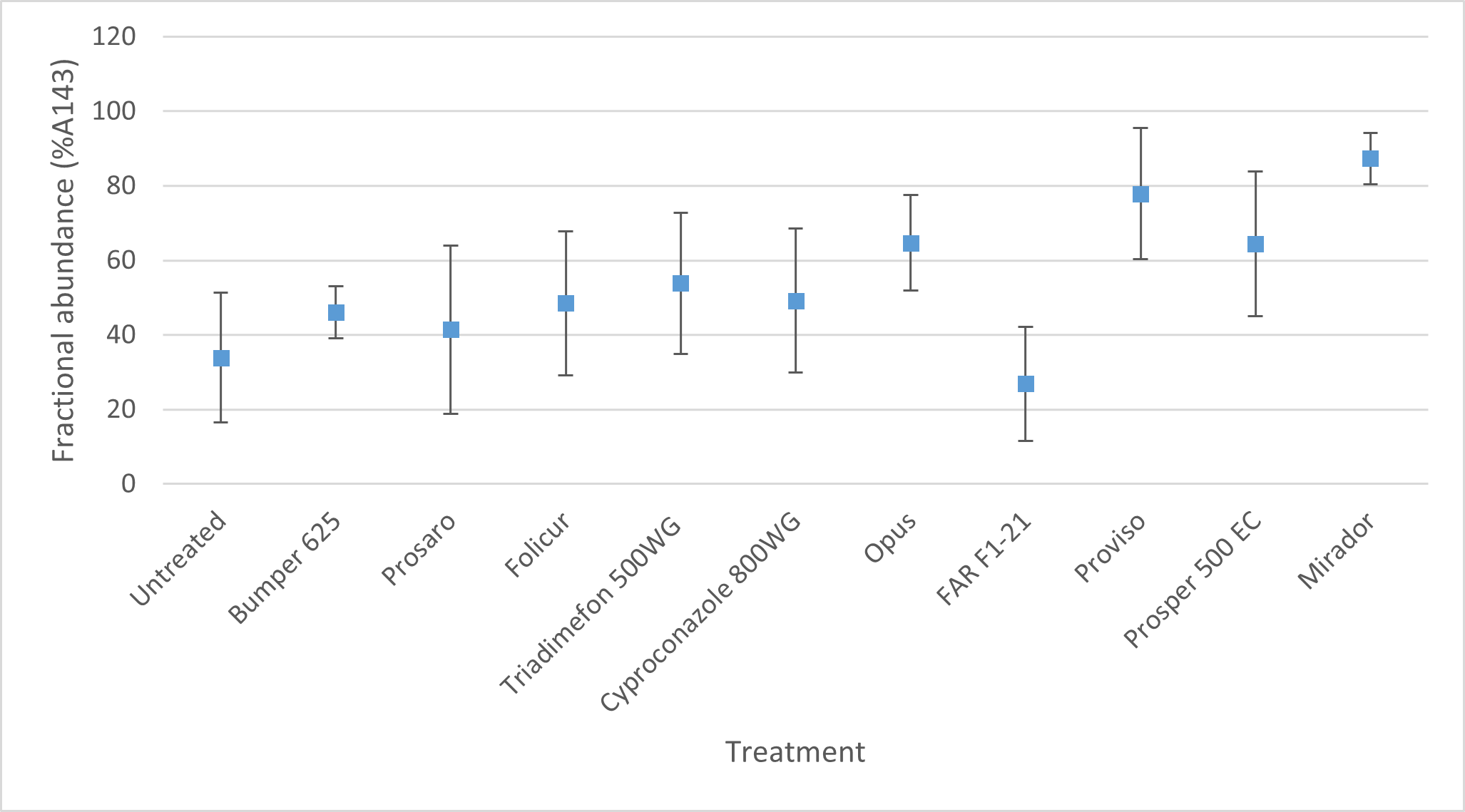 Figure 2. Fractional abundance of the A143 mutation in the different fungicide treatments applied for WPM control – cv Scepter, Katamatite, Vic 2021. (CCDM analysis)
Figure 2. Fractional abundance of the A143 mutation in the different fungicide treatments applied for WPM control – cv Scepter, Katamatite, Vic 2021. (CCDM analysis)
Note: When the mutation at G143A occurs the G amino acid in the wild type is replaced with an A amino acid
SDHI resistance and reduced sensitivity in net form of net blotch (NFNB) in barley
The SdhC-H134R mutation in the SDHI (Group 7) target site, was detected in six samples from Victoria and one sample from South Australia in 2021. This mutation was first observed in Australia in NFNB from the Yorke Peninsula of South Australia in 2019 and is associated with the highest resistance factors affecting the key SDHI compounds such as fluxapyroxad, bixafen and benzovindiflupyr.
Four other samples from Victoria and one sample from South Australia in 2021 were associated with low resistance factors for SDHI compounds and classed as the mutations conferring reduced sensitivity. These mutations have been detected previously. In the case of the SdhD-D145G mutation it was first observed in Australia in NFNB from the Yorke Peninsula of South Australia in 2019 and in the case of SdhC-N75S in spot form of net blotch (SFNB) in the Cunderdin region in WA in 2020.
DMI reduced sensitivity in net form net blotch (NFNB) in barley
The F489L-2 mutation in the DMI (Group 3) target, Cyp51A, was detected in six samples from Victoria and one sample from South Australia in 2021. This mutation was previously observed in Australia in NFNB from the Yorke Peninsula of South Australia in 2019 and is associated with reduced sensitivity to DMI compounds.
Genetic changes in the region that controls the DMI target were detected in one sample from South Australia in 2021. This different type of mutation has been previously observed in Australia in spot form net blotch (SFNB) from Western Australia since 2016 and is associated with reduced sensitivity to DMI compounds.
QoI resistance in septoria tritici blotch (STB)
Fungal cultures isolated from two STB samples collected in South Australia in 2020, were found to carry the fungicide resistance mutation A143, which is associated with full resistance to QoI (Group 11) fungicides. In vitro analysis of two STB resistant isolates obtained from these samples showed a 200-fold increase in azoxystrobin resistance compared to sensitive reference isolates. Subsequent molecular analysis of 32 STB samples collected from 29 locations across Victoria, South Australia and NSW in 2021 did not detect the mutation associated with resistance to QoI fungicides.
So what does this mean for growers and advisers
Fungicide resistance management strategies which should be used within broader IDM include:
- With wheat and barley crops where two to three fungicide applications occur within a season, avoid repeat applications of the same product/active ingredient and where possible also avoid the same mode of action in the same crop. This is particularly important when using Group 11 QoI (strobilurins) and Group 7 SDHIs, which preferably would only be used once in a growing season
- Avoid using the seed treatment fluxapyroxad (Systiva®) year after year in barley without rotating with foliar fungicides of a different mode of action during the season
- Avoid applying the same DMI (triazole) Group 3 fungicide twice in a row, irrespective of whether the DMI is applied alone or as a mixture with another mode of action
- Avoid the use of tebuconazole alone and flutriafol for Septoria tritici blotch (STB) pathogen control in regions where reduced sensitivity is problematic, as these Group 3 DMIs are more affected by reduced sensitivity strains than other DMIs
- Group 3 DMIs such as epoxiconazole (Opus®) or triazole mixtures \such as prothioconazole and tebuconazole (Prosaro®) when used alone are best reserved for less important spray timings, or in situations where disease pressure is low in higher yielding scenarios.
- With SDHI seed treatments such as fluxapyroxad (Systiva®) or QoI fungicides used in-furrow such azoxystrobin (Uniform®), consider using a subsequent foliar fungicide with a different mode of action, and therefore avoiding, if possible, a second application of SDHI or QoI fungicide active.
Clearly, the best way to avoid fungicide resistance is not to use fungicides! However, in high disease pressure regions, this would be an unprofitable decision. When a cultivar’s genetic resistance breaks down or is incomplete, it is imperative that growers and advisers have access to a diverse range of effective fungicides (in terms of mode of action) for controlling leaf disease. Hence, we need to protect their longevity. In order to protect them, one of the most effective measures is to minimise the number of fungicide applications applied during the season. Therefore, consider all aspects of an Integrated Disease Management (IDM) strategy when putting your cropping plans together at the start of the season, since this will help reduce our overall fungicide dependency.
Principle components of IDM
Rotations – where possible avoid high risk rotations for disease, for example, barley on barley or wheat on wheat.
Seed hygiene – minimise the use of seed from paddocks where there were high levels of disease that could be seedborne (e.g. Ramularia, net form net blotch).
Use less disease susceptible cultivars, particularly when sowing early. Where this is not possible delay the sowing of the most susceptible cultivars to reduce disease pressure where the phenology of the cultivar is adapted to the later development window.
Cultural control such as stubble management, where disease risks are high and the penalties for stubble removal are not as high.
Grazing early sown cereal crops up to GS30 to reduce disease pressure.
AFREN (Australian Fungicide Resistance Extension Network)
The Australian Fungicide Resistance Extension Network (AFREN) was established to develop and deliver fungicide resistance resources for grains growers and advisers across the country. It brings together regional plant pathologists, fungicide resistance experts and communications and extension specialists.
AFREN wants to equip growers with the knowledge and understanding that they need to reduce the emergence and manage the impacts of fungicide resistance in Australian grains crops.
As members of AFREN, the authors of this paper are keen to hear if you believe you are encountering reduced sensitivity or resistance in your broad acre crops.
Acknowledgements
FAR Australia would like to acknowledge the assistance of Andrew McPherson and James Reilly in sourcing and managing the field trial conducted near Katamatite in 2021. The research undertaken as part of this project is made possible by the significant contributions of growers and their advisers through their support of the GRDC.
Contact details
Nick Poole, FAR Australia
Business Address: Shed 2/63 Holder Rd, Bannockburn, Victoria 3331
Ph: 08 5266 1290 / 0499 888 066
Email: nick.poole@faraustralia.com.au
Fran Lopez-Ruiz
Centre for Crop and Disease Management, Curtin University, Perth, WA 6845
Ph: 08 9266 3061
Email: fran.lopezruiz@curtin.edu.au
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
GRDC Project Code: CUR1403-002BLX, CUR1905-001SAX,
Was this page helpful?
YOUR FEEDBACK
