Harvest height implications for Fusarium crown rot management
Author: Toni Petronaitis (NSW Department of Primary Industries, Tamworth, NSW & University of New England, Armidale, NSW), Clayton Forknall (DAF Qld), Steven Simpfendorfer (NSW DPI), Richard Flavel (UNE) and David Backhouse (UNE) | Date: 02 Mar 2022
Take home messages
- Taller standing stubble allowed vertical progression of the Fusarium crown rot fungus within the stubble after harvest, whilst short stubble prevented further growth (i.e. vertical growth was limited to the height of the cut stubble).
- Stripper fronts, which leave higher standing stubble, may increase stubble-borne disease inoculum after harvest of an infected crop, especially if wet fallow conditions are experienced.
- In high-risk situations, such as an infected crop with high biomass, cutting the crop shorter at harvest will limit further inoculum development within the stubble after harvest (beyond the levels already present at harvest).
- Cutting infected cereal stubble shorter prior to rotation with shorter-stature crops such as chickpea or lentils also prevents the dispersal of infected stubble when harvesting these shorter break crops.
Introduction
Despite continuous research and the development of crop protection strategies, the impacts of Fusarium crown rot (FCR), caused by the fungus Fusarium pseudograminearum (Fp), have increased in Australia over the past four decades. The adoption of conservation-agriculture practices such as cereal stubble retention helps to offset the risk of low in-crop rainfall but promotes the carry-over of Fp inoculum to successive cereal crops (Simpfendorfer and McKay, 2019). Despite the yield penalties associated with FCR, the benefits of cereal stubble retention on soil structure, moisture and fertility are considered a necessity in the northern grain’s region (NGR, northern New South Wales and Queensland). Finding ways to limit the negative effects of disease whilst retaining cereal stubble is therefore important to crop production in the NGR.
The adoption of higher harvest-heights (stripper-fronts), light tillage (Kelly-chaining) and rotations with shorter stature break crops such as chickpea (Cicer arietinum) are becoming common in the NGR. Stripper front harvesting systems improve harvest efficiency through the rapid ‘stripping’ of heads during harvest, but also increases retained standing stubble biomass by increasing standing stubble height i.e., ~50-60 cm compared to ~30 cm with a combine harvester. It is unknown how such an increase in vertical cereal stubble height will affect the survival and/or growth of Fp.
Fusarium pseudograminearum is capable of surviving in post-harvest cereal stubble for ~3 years (Summerell and Burgess 1988)and can also continue to colonise (grow) in post-harvest cereal stubble (Petronaitis et al. 2020) by a process known as saprotrophic colonisation. Additional cereal stubble remaining from stripper front-harvests may increase the opportunity for saprotrophic colonisation, as there is more cereal stubble to vertically colonise, compared to the extent of growth possible in stubble remaining from conventional or shorter harvest-heights. This has the potential to increase inoculum levels and inoculum dispersal. As such, lowering of the harvest-height of a cereal crop infected with Fp may restrict saprotrophic colonisation of standing cereal stubble after harvest. If true, reducing or modifying harvest-heights of cereals infected with FCR could be beneficial for preventing further increases in Fp inoculum levels during fallow or non-host periods.
What did we do?
Field experiments were conducted at Breeza and Narrabri in northern New South Wales, spanning the 2019, 2020 and 2021 winter crop growing seasons. Cereal stubble (from durum wheat of the variety DBA Lillaroi) with extensive Fp colonisation was established at both sites in 2019 and a range of target harvest-height (low, medium or high) and harvest-trash (trash returned to plot or trash removed off plot) treatments were imposed at harvest in 2019. Prior to sowing in 2020, an additional stubble management treatment (Kelly-chain) was imposed on a selection of plots. This treatment was applied in combination with the harvest-height treatments, to plots that had previously had trash retained. A chickpea break crop (PBA Seamer) was subsequently sown across both field experiments in 2020.
Chickpea plant populations (plants/m2) of variety PBA Seamer were counted in each plot 30 days after planting. Lowest pod heights were measured on two random plants per plot prior to harvest as the distance from ground level to lowest pod. Grain yield was determined from machine harvested grain samples taken from 2 × 10 m plots.
Soil moisture content (SMC) was measured in November 2019, May 2020 and November 2020. One 1.2 metre soil core was sampled per plot and cut into 0-30 cm, 30-60 cm, 60-90 cm and 90-120 cm segments. The wet weight and dry (dried for 48 hours at 105 °C) weight of each soil segment was measured to calculate gravimetric SMC.
Durum stubble from 30 plants were collected at random across each plot in November 2019 (durum harvest), May 2020 (chickpea sowing) and November 2020 (chickpea harvest). Stubble was separated into individual tillers and twenty tillers were then selected randomly for culturing. Starting at the stem base (crown), a 1.5 cm segment was removed from the tiller every 5 cm along the entire tiller length. Stem portions were surface sterilised (5 mL sodium hypochlorite solution, 45 mL deionised water, 50 mL >98% ethanol) for 1 minute then washed with sterile water. Samples were dried overnight and plated on 1/4 strength potato dextrose agar (PDA) + novobiocin (10 g PDA, 15 g technical agar plus 0.1 g novobiocin/L water) and incubated under alternating ultra-violet light (12 h light/12 h dark) for 7 days at 25 °C. Pathogen incidence was recorded as the number of segments producing typical Fp colonies based on morphology. Maximum colonisation was defined as the maximum height at which Fp was detected in each sample.
The nine stubble management treatments (factorial combination of harvest-height and harvest-trash, plus Kelly-chain treatments), were randomly assigned to plots in each experiment according to a randomised block design, with three replicate blocks. The response variable, length of maximum colonisation, was analysed across sampling times, for each experiment separately using a linear mixed model framework, whereby treatments, sampling time and their interaction were fit as fixed effects while structural terms were fit as random. The analysis of SMC used a similar modelling approach with the treatment structure expanded to include a fixed effect corresponding to the depth of sampling, and the subsequent interaction effects between depth, treatments and sampling time. Response variables related to chickpea crop performance were analysed separately for each experiment. All models were fit using the ASReml-R package in the R statistical computing environment.
What did we find?
Saprotrophic colonisation of cereal stubble by Fp was restricted in shorter stubble
The maximum colonisation height of Fp in the post-harvest cereal stubble increased significantly over the 2019-20 fallow in the medium (32 or 25 cm) and tall (48 or 38 cm) stubble at both sites (P < 0.001, Figure 1). Fp height did not change in the short (17 or 13 cm) stubble because the fungus had already reached the observed (cut) height at harvest (Nov 2019). At Breeza, maximum colonisation height increased significantly in medium (+11.1 cm) and tall (+22.2 cm) stubble over the fallow period from Nov 2019 to May 2020 (Figure 1). Similarly, at Narrabri, Fp progressed significantly in medium (+15.2 cm) and tall (+21.4 cm) stubble over the same period (Figure 1). Maximum colonisation then decreased slightly over the chickpea break crop period (from May 2020 to Nov 2020) but was still elevated significantly in the medium and tall stubble compared with the shorter stubble heights at both sites (Figure 1).
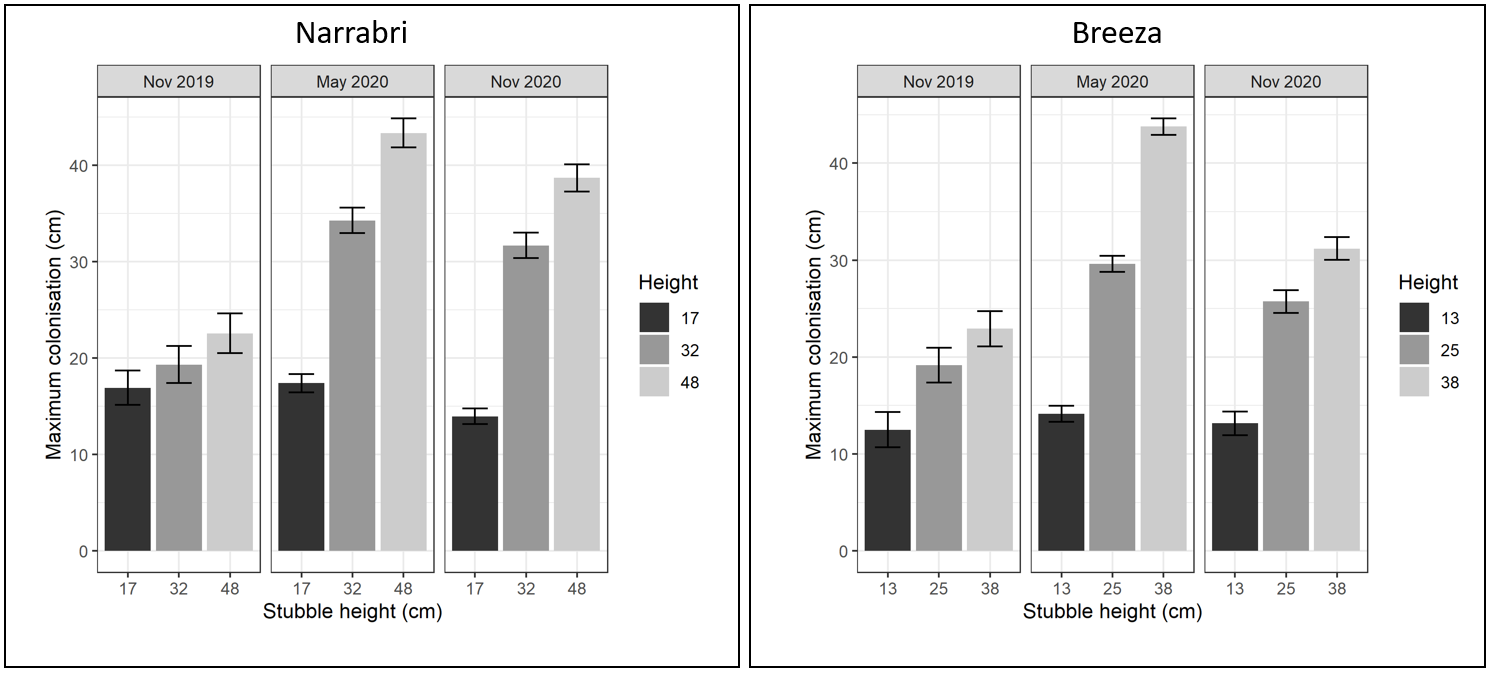
Figure 1. Maximum vertical colonisation by Fusarium pseudograminearum in cereal stubble of different heights (mean observed height, in cm) from harvest of the infected crop (Nov 2019), a summer fallow (May 2020) and a chickpea break crop (Nov 2020) at Breeza and Narrabri in NSW. Note harvest-heights were unique to each site due to differences in final crop height in 2019, with slight variability in actual height achieved between and across plots for each target height treatment. Error bars represent the approximate back-transformed standard error of the mean.
Maximum colonisation of short stubble at Breeza in November 2019 was significantly lower than medium and tall stubble, but this was possibly a reflection of the shorter stubble treatment imposed (stubble was sampled after harvest), given that maximum colonisation at the Narrabri site was more uniform (Figure 1). Maximum colonisation measurements above the mean observed height (e.g., Breeza in May 2020), was due to variation in individual tiller lengths within a harvest-height treatment (Figure 1). There was no effect of cereal trash treatment (retained, removed or Kelly-chained) on maximum colonisation at each time of sampling for both sites (P > 0.1).
These results demonstrate that Fp can continue to saprotrophically colonise cereal stubble after harvest. Specifically, if stubble is left longer, Fp can colonise to the cut height of cereal stubble in the first six months after harvest and persist high within the stem for at least another six months (compared with levels at harvest in November 2019). These findings support the concept that lower cereal harvest-heights are effective at preventing the vertical progression of Fp in infected standing stubble post-harvest.
Cereal stubble treatments did not compromise soil moisture
There were no detrimental effects of the cereal stubble treatments on soil moisture levels after the 2019 summer fallow (May 2020) and after harvest of the chickpea crop (November 2020) (P > 0.2) (data not shown). There was good fallow rainfall at both sites: 324 mm at Narrabri and 439 mm at Breeza (from 01/12/19 to 31/05/20), significantly increasing soil moisture over the fallow period (for depths 0 to 90 cm, P < 0.03). So although the stubble treatments didn’t affect fallow efficiency at these sites, the different stubble treatments may have had a more profound impact on soil moisture levels if drier conditions had persisted over summer and autumn.
Chickpea crop performance was not affected by cereal stubble treatments
Overall, the cereal stubble treatments did not have any meaningful impact on chickpea performance in these experiments, with no differences in yield, and only minor differences in chickpea establishment. There was no significant effect on chickpea yield of standing stubble height (P > 0.96), trash treatment (P > 0.19) or the interaction of harvest-height and trash treatments (P > 0.14) at both sites (data not shown). At Breeza, the Kelly-chained treatment resulted in slightly higher chickpea establishment (+4 plants per m2) compared to the trash retained treatment (P = 0.05), possibly due to better seed-soil contact when using a disc seeder in Kelly-chained plots. Lowest pod height was not affected by cereal stubble treatments at either site (P > 0.32).
Implications for stripper front harvest adoption
The present study confirms that Fp can saprotrophically colonise the full length of cereal stubble in the field, given sufficient fallow rainfall. Harvesting higher with a stripper front may therefore increase risk of higher Fp inoculum levels compared harvesting at a lower height with a conventional combine header. Given that Fp is detected in 100% of cereal crops in New South Wales (with majority in the ‘high’ category) (Milgate and Simpfendorfer, 2020), the widespread use of stripper fronts could result in further increases in disease incidence and severity in this region. Planning for stubble management (including stubble/harvest heights) prior to harvest, based on the infection status of the cereal crop to be harvested and future crop sequence, is therefore recommended.
In cereal crops infected with Fp, reducing stubble height by harvesting lower would be a useful strategy to limit saprotrophic colonisation after harvest. Ideally, harvest height would be above the height at which the stubble has already been colonised by Fp, as this means that less infected stubble is spread into the inter-row spaces, thus optimising inter-row sowing strategies to minimise disease in subsequent cereal crops. This approach could still be used with stripper-fronts by stripping grain, if desired, then following up with a shorter harvest height. The cut fraction (free of pathogen) could be left between rows as mulch or baled and removed. If saprotrophic colonisation has occurred during a wet summer period, cutting low, baling and removing the infected stubble prior to sowing the next crop is preferred to burning stubble. This way there is still a proportion of ground cover to protect the soil surface, but the bulk of inoculum that may infect the next crop has been removed.
Restricting movement of Fp vertically within standing cereal stubble may provide two-fold benefits. Firstly, it can prevent inoculum build-up within the standing stubble fraction, beyond the inoculum levels present at harvest. Secondly, it may stop the spread of inoculum across a paddock during harvest of short-stature crops such as chickpea, improving the efficacy of inoculum avoidance strategies like inter-row sowing. Harvesting cereals above the height of Fp colonisation could prevent the non-colonised stubble fraction from becoming saprotrophically colonised. Although the cereal harvest-height modification for FCR management appears promising, the implications on FCR risk in a subsequent cereal crop are still to be determined in these field experiments in 2021 (results not available at time of writing).
Stripper fronts offer faster and more efficient crop harvest but could potentially create future issues in cereal crops infected with Fp. Even if only low levels of infection are experienced during the growing season, or disease expression is restricted (stem browning/whiteheads) by favourable seasonal conditions or plant tolerance, rapid colonisation of stubble may still occur after plant senescence (Petronaitis et al. 2020). So, be vigilant about checking your cereal crops for disease symptoms and consider confirmation of inoculum levels and hence risk through diagnostic services if necessary.
Testing using PREDICTA® B is effective in determining disease risk (following the up-to-date protocol of adding cereal stubble to the sample). If your paddock/s have returned a below detection limit or low risk PREDICTA® B test for cereal disease, then you can continue following best practise agronomy for your next cereal crop.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC and the authors would like to thank them for their continued support. Ms Petronaitis would like to thank the GRDC and NSW DPI for co-funding her GAPP PhD scholarship (BLG211). Technical support provided by Chrystal Fensbo, Alana Johnson, Luke Neale, Finn Fensbo, Jason McCulloch, Stephen Morphett, Michael Dal Santo and Jim Perfrement is gratefully acknowledged.
References
Milgate, A, and Simpfendorfer, S (2020). Pathogen burden in NSW winter cereal cropping. GRDC Update Paper. Accessed 09-12-21
Petronaitis T, Forknall C, Simpfendorfer S, Backhouse D (2020) Stubble Olympics: the cereal pathogen 10cm sprint. GRDC Update Paper. Accessed 09-12-21
Simpfendorfer, S and McKay, A (2019). What pathogens were detected in central and northern cereal crops in 2018? GRDC Update, Goondiwindi, 106-115
Summerell BA and Burgess LW (1988) Stubble management practices and the survival of Fusarium graminearum Group 1 in wheat stubble residues. Australasian Plant Pathology 17:88-93
Contact details
Toni Petronaitis
NSW DPI
4 Marsden Park Road, Tamworth NSW 2340
Ph. 02 6763 1219
Email: toni.petronaitis@dpi.nsw.gov.au
Twitter: @ToniPetronaitis
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
® Registered trademark
Was this page helpful?
YOUR FEEDBACK
