The impact of Ascochyta on chickpea yield and economics when infection occurs at three different growth stages
The impact of Ascochyta on chickpea yield and economics when infection occurs at three different growth stages
Author: Hayley Wilson (NSW DPI, Tamworth), Leigh Jenkins (NSW DPI Trangie), Steven Harden (NSW DPI, Tamworth), Kevin Moore (NSW DPI, Tamworth) | Date: 25 Feb 2022
Take home message
- Variety choice remains a critical management tool under high disease pressure (gross margin loss of $300/ha in Kyabra compared to gains of up to $1000 in PBA Seamer when no fungicide applied)
- Preventative application of fungicide before seedling infection has the greatest impact in reducing severity of disease
- Salvage application of fungicide to seedling infection in susceptible varieties is insufficient in preventing yield loss
- Seasonal conditions in 2021 were not conducive to determine if infection during the vegetative growth stage impacts yield
- Application of fungicide during early podding growth stage may reduce yield loss if Ascochyta blight is present and a wet-season finish is predicted.
Introduction: why was this research done?
Ascochyta blight (AB) management in chickpea across southern Queensland and north-central New South Wales regions is based on controlling early season infection. This strategy has been developed through agronomist feedback and past Tamworth Agricultural Institute (TAI) experiments where infection is simulated after the first post emergent rain event. However, dependant on varietal resistance, chickpeas are also known to be susceptible to AB infection later in the season during flowering and podding. Limited studies have been conducted on the impact of AB management when infection occurs during flowering and podding stages of chickpea in Australia.
In 2020 an experiment was conducted at Trangie Agricultural Research Centre (TARC). This established that early disease management and higher AB varietal resistance continues to be the most profitable management strategy when compared to uncontrolled infection with susceptible varieties. However, the 2020 results raised further questions about what would occur if fungicide application was reactive after the first post emergent rainfall event, or if no fungicide was applied until pod infection. These treatments were added to the 2021 experiments conducted both at TARC and TAI to assess the economic impact of AB infection at three separate growth stages on chickpea varieties with different levels of AB resistance.
Methods
Variety and AB resistance
- Kyabra VS = Very susceptible
- PBA HatTrick MS = Moderately susceptible
- PBA Seamer MS = Moderately susceptible
Treatments applied per variety
- No disease (LOW): Plots un-inoculated with AB and any potential disease controlled using foliar chlorothalonil fungicide applied before rain or irrigation events
- High disease (HGH): Plots inoculated with disease twice at seedling (3-4 nodes) and vegetative (7-8 nodes) growth stage with no fungicide applied
- Seedling 1 (SDG1): Plots inoculated with AB at seedling stage (3-4 nodes), with one prior (preventative) fungicide treatment at 2-3 node stage. Allow disease to progress for 2-3 rain events, then control for remainder of season with foliar fungicide as with LOW treatment
- Seedling 2 (SDG2): Plots inoculated with AB at seedling stage (3-4 nodes), with no prior (preventative) fungicide treatment. Allow disease to progress for 2-3 rain events, then control for remainder of season with foliar fungicide as with LOW treatment
- Vegetative (VEG): Plots protected with foliar fungicide from emergence to vegetative growth stage then inoculate with AB. Allow disease to progress for 2-3 rain events or to first pod, then control again with foliar fungicide for remainder of season
- Podding 1 (POD1): Plots are protected with foliar fungicide from emergence to first pod then inoculated with AB, disease allowed to progress through to harvest
- Podding 2 (POD2): Plots progress to the podding stage without foliar fungicide protection then inoculated with AB, disease allowed to progress through to harvest
Fungicide application and infection events
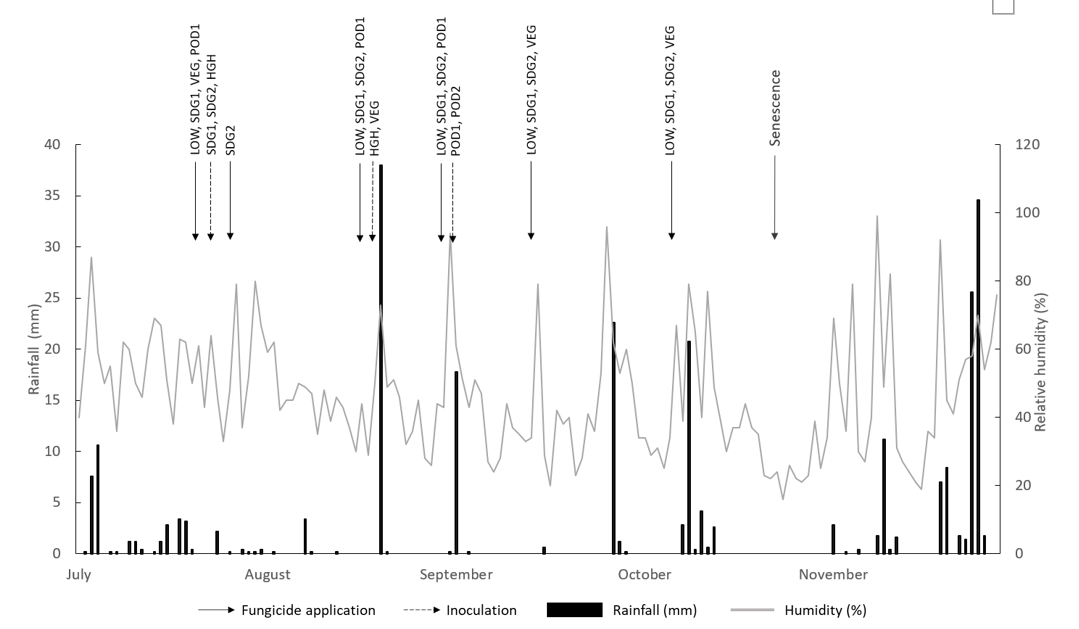
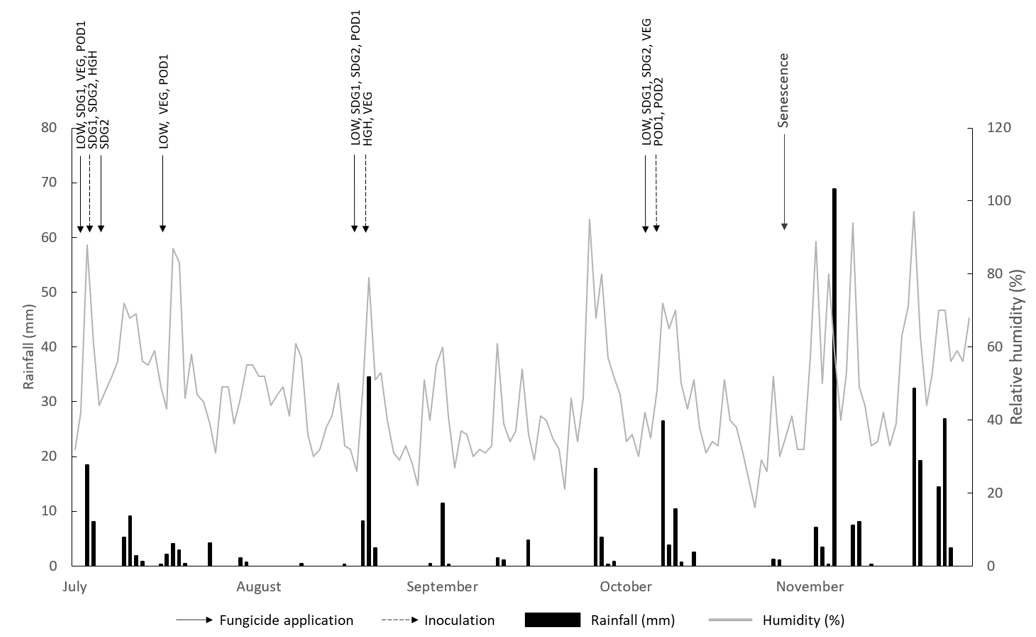
All fungicide treatments were chlorothalonil (720 g/L) @ 1 L/ha with up to 6 treatments applied to LOW disease plots. Inoculation was completed with AB conidial suspension @ 400,000 – 300,000 conidia/mL. Additionally, two AB-infected spreader plants per inoculated plot were planted at each end of the treatment plots at each growth stage timing for infection. Treatments were applied at each site according to predicted rainfall that would sustain 6-12 hours of leaf wetness (Figure 1 and 2).
Field experimental design and operations
Field experiments were conducted at TARC and TAI on grey and light clay soil respectively. The experiments were sown in a randomised block design on 16and 29 of May at TAI and TARC respectively. All chickpea varieties were sown at 35 plants/m2 on 30 cm row spacing with Pulse starter Z fertiliser and rhizobia group N. All experimental seed was treated with P-Pickel T® pre sowing. Field experiments were managed according to best practice weed and insect management. Pre-harvest desiccation was applied using Reglone® (200 g/L diquat) @ 2 L/ha at TAI; and Roundup Ultra®MAX (570 g/L glyphosate) @ 1.7 L/ha plus Sharpen® (700 g/kg saflufenacil) @ 34 g/ha at TARC. The experiments were harvested to assess grain yield response on 13 December at TARC and 19 December at TAI.
Data collection and analysis
Severity of AB was assessed on a 1 – 9 scale (Table 1). Treatment HGH represented the positive control for each variety (high disease – no fungicide applied) and LOW represented the negative control (low disease – multiple fungicides applied) in comparison to all other treatments. The TAI trial was scored on August 17, September 29 and October 25. The TARC trial was scored on September 13, September 28 and October 27. Gross margin was calculated based on the PIRSA gross margin for chickpeas.
Table 1. Summary of Ascochyta blight disease scale
No. score | Definition |
|---|---|
1 | Disease symptoms not detected |
2 | Leaf lesions on the lower canopy are rare, no leaf lesions on the upper canopy |
3 | Leaf lesions on the lower canopy are rare, leaf lesions on the upper canopy rare |
4 | Leaf lesions on the upper canopy common |
5 | Stem lesion rare, leaf lesions on upper canopy common |
6 | Stem lesions uncommon, leaf lesions on upper canopy common |
7 | Stem lesions common, leaf lesions on upper canopy common, stumps uncommon |
8 | Stem lesions common, leaf lesions on upper canopy common, stumps common |
9 | All plants are dead |
Results
Disease severity
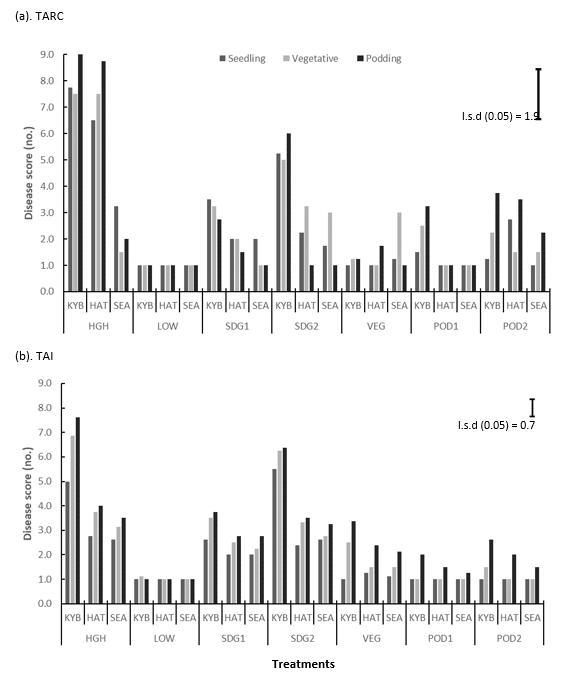
Disease assessments were taken at least two weeks after disease inoculation and application of corresponding fungicide management treatments. The no fungicide (positive control (HGH)) treatments for susceptible variety Kyabra scored highest for disease severity at both TARC and TAI. For moderately susceptible varieties PBA HatTrick and PBA Seamer at TARC the highest scores were ≥ 8 and ≥ 3 respectively. The same varieties at TAI scored ≥ 4 and ≥3 respectively. At both TARC and TAI there was little to no disease for the full fungicide (negative control (LOW)) treatments (Figure 3). Overall, TARC appeared to have consistently higher disease scores than TAI.
Across fungicide management treatments at both TARC and TAI Kyabra plots with seedling infection and no early fungicide (SDG2) had the highest disease scores with significant increases in disease severity compared to the full fungicide (negative control (LOW)) treatment. At both TARC and TAI, there was a significant difference between the Kyabra SDG1 and SDG2 treatments at all assessment timings with lower disease severity in the SDG1 treatment which had a fungicide applied at the 2-3 node stage prior to inoculation at 3-4 nodes. All treatments had significantly decreased disease severity compared with the no fungicide treatments with except for TAI SDG2 which retained high severity and all PBA Seamer which remained low regardless of treatment. In comparison to the negative control (LOW), disease severity was significantly higher with the SDG1 treatment at both locations and SDG2 and VEG at TAI. In addition, disease severity was significantly different at both locations between both the negative control (LOW), POD1 and POD2, as well as between POD1 and POD2 at TARC (Figure 3).
Yield
Significant yield differences were recorded between treatments at both TARC and TAI. In the no fungicide (positive control (HGH)) treatments, there was a yield loss for Kyabra, PBA HatTrick and PBA Seamer of 97, 51 and 18 % respectively at TARC. Losses were similar at TAI with Kyabra, PBA HatTrick and PBA Seamer at 97, 60 and 18% when no fungicide was applied. When SDG1 and SDG2 treatments were compared to the negative control (LOW) where disease was not present, the greatest loss was 35% and 90% for Kyabra at TARC and TAI respectively. This is compared to moderately susceptible varieties PBA HatTrick and PBA Seamer that lost 3-8% yield at TARC and 37% and 0% at TAI. There were no differences in yield loss between the negative control (LOW) and VEG treatments. However, at TARC there was significant difference observed between the negative control, POD1 PBA Seamer , and POD2 for all varieties with losses of up to 18% (Table 2).
Table 2. Effect of Ascochyta blight infection timing on grain yield (t/ha) at TARC and TAI in 2021.
Treatment | Fungicide management strategy | Variety | TARC Yield (t/ha) | TAI |
|---|---|---|---|---|
HGH | No fungicide | Kyabra | 0.10 | 0.08 |
PBA HatTrick | 1.60 | 0.86 | ||
Seamer | 2.80 | 1.22 | ||
LOW | Every rainfall | Kyabra | 3.50 | 2.51 |
PBA HatTrick | 3.30 | 2.17 | ||
Seamer | 3.40 | 1.92 | ||
SDG1 | Before infection | Kyabra | 3.40 | 1.45 |
PBA HatTrick | 3.00 | 1.90 | ||
Seamer | 3.10 | 2.21 | ||
SDG2 | After infection | Kyabra | 2.30 | 0.25 |
PBA HatTrick | 3.20 | 1.38 | ||
Seamer | 3.10 | 1.93 | ||
VEG | Before & after infection | Kyabra | 3.50 | 2.01 |
PBA HatTrick | 3.10 | 1.80 | ||
Seamer | 3.10 | 1.85 | ||
POD1 | Before pod infection | Kyabra | 3.60 | 1.86 |
PBA HatTrick | 3.10 | 2.17 | ||
Seamer | 3.00 | 1.50 | ||
POD2 | No fungicide | Kyabra | 3.10 | 1.76 |
PBA HatTrick | 2.70 | 1.66 | ||
Seamer | 3.00 | 1.78 | ||
p-value | <0.001 | 0.005 | ||
l.s.d (P = 0.05) | 0.361 | 0.759 | ||
Gross margin
The greatest economic loss occurred at both locations when AB was not controlled at any growth stage (HGH) in the susceptible variety Kyabra (> $300 loss) (Table 3). The highest gross margin was calculated to be for the Kyabra POD1 treatment ($1,365) at TARC where disease was controlled until podding infection. The greatest discrepancies at both locations in gross margin between non-control treatments occurred when susceptible varieties were subjected to early season infection and fungicide management strategies with a > $500 difference between Kyabra SDG1 and SDG2.
Table 3. Collective effect of Ascochyta blight on gross margin across 2020 and 2021 at TARC and TAI.
Treatment | Variety | Gross margin $/ha* | ||
|---|---|---|---|---|
TARC 2020 | TARC 2021 | TAI 2021 | ||
HGH | Kyabra | -300 | -334 | -348 |
PBA HatTrick | -160 | 413 | 42 | |
Seamer | 842 | 1010 | 221 | |
LOW | Kyabra | 701 | 1287 | 822 |
PBA HatTrick | 678 | 1187 | 653 | |
Seamer | 857 | 1237 | 527 | |
SDG1 | Kyabra | - | 1237 | 292 |
PBA HatTrick | - | 1038 | 516 | |
Seamer | - | 1087 | 673 | |
SDG2 | Kyabra | -399 | 689 | -305 |
PBA HatTrick | 174 | 1137 | 258 | |
Seamer | 843 | 1087 | 534 | |
VEG | Kyabra | 506 | 1301 | 690 |
PBA HatTrick | 518 | 1102 | 585 | |
Seamer | 934 | 1102 | 608 | |
POD1 | Kyabra | 862 | 1365 | 496 |
PBA HatTrick | 765 | 1116 | 651 | |
Seamer | 898 | 1066 | 318 | |
POD2 | Kyabra | - | 1159 | 490 |
PBA HatTrick | - | 959 | 440 | |
Seamer | - | 1109 | 500 | |
* Gross margin based on December 2021 chickpea price of $498 per tonne, fungicide application cost of $14.25 per ha and other contributing production costs of $385.15 per ha; based on 2021 PIRSA gross margin calculator for chickpea crops.
Discussion
This experiment demonstrated that early seedling AB infection had the greatest impact on disease severity but was reduced by fungicide application before the initial rainfall event for susceptible varieties. However, where early seedling infection did occur it did not translate to significant yield loss in varieties with a moderately susceptible disease rating. The benefits of using varieties with increased resistance is their ability to recover yield regardless of disease severity earlier in the season once fungicide is applied as shown by the PBA HatTrick (37%) SDG2 treatment. This recovery of PBA HatTrick yield at TARC, contrary to higher disease scores, may also be in response to mild spring conditions and additional rainfall in September (Figure 1). This recovery was also mirrored by the PBA Seamer positive control (HGH) treatment (Figure 3). Both these results indicate that varieties with a higher AB resistance rating are more likely to recover yield once AB is controlled after a seedling infection event provided seasonal conditions are conducive for grain yield recovery.
Unfortunately, no conclusion can be made regarding AB management at the vegetative stage. Firstly, the infection events for the VEG treatments did not have sufficient rainfall and spreading events post infection (Figure 1) to result in a yield loss. Secondly, during the vegetative stage of growth there is increased vigour in plant growth making chickpeas less susceptible to infection at this time. Reduced AB disease severity with infection at the vegetative growth stage has been found in a similar Indian study (Basandrai et al., 2007). This indicates that a missed fungicide application during the vegetative stage of chickpea growth is not as likely to cause yield damage as with seedling AB infection. Further testing of infection at this growth stage is required across more locations and years to clarify this under Australian chickpea growing conditions.
Anecdotal evidence suggests that podding infection is likely to have yield and seed quality impacts as plant resistance plateaus during this growth stage. However, under Australian conditions, podding normally occurs during mid to late spring when rainfall generally declines after the winter growing season. Hence, if AB has been adequately controlled in-crop throughout the winter growing season, then economic return from late season podding control is likely to be dependent on the continuing seasonal weather forecast. For example, in the experiment at TARC in 2020, the gross margin for protecting against AB at podding had minimal economic benefits under dry spring conditions which were unfavourable for AB infection (Moore et al., 2021). In contrast the experiment at TARC in 2021 showed plants which were protected from AB at podding during the wet finish displayed increased yield response and justifiable economic benefit from fungicide application.
Variation in gross margin between sites and years is important to note. Indicating strongly that input of fungicide and varietal choice will have returns dependent upon site location and weather conditions. Therefore, seasonal planning to maximise predicted income according to long range weather forecasts through variety, paddock location and fungicide regime choice is recommended.
Conclusion
The choice of a variety with improved AB resistance remains the most important tool for protection against yield loss in chickpea. The application of a fungicide prior to the initial rainfall event at the seedling stage will have the greatest impact on reducing disease severity However, moderately susceptible varieties are able to maintain yield potential if that initial seedling fungicide application is missed for some reason. No conclusions can yet be made regarding AB infection at the vegetative growth stage. Fungicide application may be required at early podding if AB is detected at this growth stage and wet seasonal conditions are predicted to protect against yield and economic loss.
References
Basandrai AK, Basandair D, Pande S, Sharma M, Thakur SK, Thakur HI (2007) Development of Ascochyta blight (Ascochyta rabiei) in chickpea as effected by host resistance and plant age. European Journal of Plant Pathology, 119, 77-86.
Moore K, Jenkins L and Harden S (2021) The economics of managing Ascochyta in chickpea when disease occurs at different growth stages and implications for spray timing. Dubbo GRDC Grains Research Update, 2021
PIRSA, SARGIT, GRDC (2021) Farm gross margin and enterprise planning guide.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support.
Hayley Wilson and Leigh Jenkins would also like to acknowledge the significant contribution of growers and agronomists to supporting this research. They would also like to thank technical support provided at NSW DPI TARC and TAI locations by Scott Richards, Paddy Steele, Timothy McNee, Paul Nash and Gail Chiplin. Dr Georgina Pengilley, Dr Steven Simpfendorfer and Dr Kurt Lindbeck are also acknowledged for their contributions to editing and review of this paper.
Contact details
Hayley Wilson
Department of Primary Industries – Tamworth Agricultural Institute
4 Marsden Park Road, Calala, NSW, 2340
Ph: 0438 729 682
Email: hayley.wilson@dpi.nsw.gov.au
Leigh Jenkins
Department of Primary Industries – Trangie Agricultural Research Centre
7878 Mitchell Highway, Trangie, NSW, 2823
Ph: 0419 277 480
Email: leigh.jenkins@dpi.nsw.gov.au
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
GRDC Project Code: DAN00213, DPI1805-018BLX,
