Timing of flowering and pod initiation influences yield potential in chickpeas
Timing of flowering and pod initiation influences yield potential in chickpeas
Author: Neroli Graham (NSW DPI, Tamworth), Rosy Raman (NSW DPI Wagga Wagga), Annie Warren (NSW DPI Tamworth) and Muhuddin Anwar (NSW DPI Wagga Wagga) | Date: 25 Feb 2022
Take home messages
- Chickpeas are sensitive to average temperatures of less than 15°C during flowering, which causes flower abortion
- Early flowering does not equal early pod set or increased grain yield
- Sow within the recommended cultivar window to ensure that the majority of flowering occurs when temperature conditions are optimal
- Understanding developmental phase changes can improve productivity by aligning flowering and podding with optimal temperatures for a given environment.
The story so far
Chickpeas are suited to environmental conditions of many grain growing regions of Australia and are an important winter pulse crop for farming systems. Despite this, in northern NSW and southern Queensland, abiotic and biotic factors are estimated to cause yield reductions of 1.7 – 2.7 t/ha (Yield Gap Australia, 2018). In 2016, cool spring weather was estimated, to have resulted in yield losses of 0.5 – 0.7 t/ha in northwest NSW (K. Hobson, pers. com.).
Under ideal environmental conditions, chickpea will produce pods within a couple of days of flowering (Clarke & Siddique, 1998). However, if temperatures stay below optimal levels the length of time between the beginning of flowering and the commencement of podding can be more than two months (Berger et al., 2005). Furthermore, if average daily temperatures are below 15°C during flowering, there is reduced pollen viability, delayed stigma development and flower drop as reported by Siddique and Sedgley (1986) and Srinivasan et al. (1999). They observed that average temperatures during flowering significantly influenced flower abortion. Siddique and Sedgley (1986) found that when chickpeas were sown early and experienced average temperatures of 12.5°C during flowering there was 800 aborted flowers/m2, compared to 0 aborted flowers/m2 from a later sowing date where the average temperature was 16.8°C during flowering.
How did we get here?
In 2020, detailed phenology experiments were conducted at Tamworth in northern NSW and Wagga Wagga in southern NSW. In this series of experiments, sowing time was used to assess the capacity of a range of genotypes to produce pods and seeds under cool temperatures. These experiments included a set of 12 diverse genotypes, both released cultivars and advanced breeding lines, and targeted three sowing windows, with an early (late April to early May), main (mid-May to early June) and late season (mid to late June) sowing. Plant phasic development stages, between late vegetative and pod initiation were defined and dates were recorded. Changes in phase development that are important for pod and seed development under suboptimal environmental conditions included; the date when plants become reproductive, the date when 50 % of plants have one open flower and the date of pod initiation.
What did we find?
Phenology responses – flowering and pod initiation
Sowing date and location were found to influence the timing of phasic development of individual genotypes. At Tamworth, from the early sowing, in late April (Figure 1), the early flower CBA line had at least one flower on 50% of plants by 28 July, 22 days earlier than the next cultivar, CBA Captain and 35 days earlier than the slowest variety Kyabra. When sown in late April, Kyabra flowered on 1 September, 2 days before the average air temperature rose above 15°C on 3 September (Figure 1). In contrast, when the cultivars were sown in the optimal/main sowing window in late May, the early flower CBA line commenced flowering on 29 August, with CBA Captain and PBA Striker achieving 50 % flowering on 5 September, and Kyabra and PBA Seamer flowering on 8 September (Figure 1).
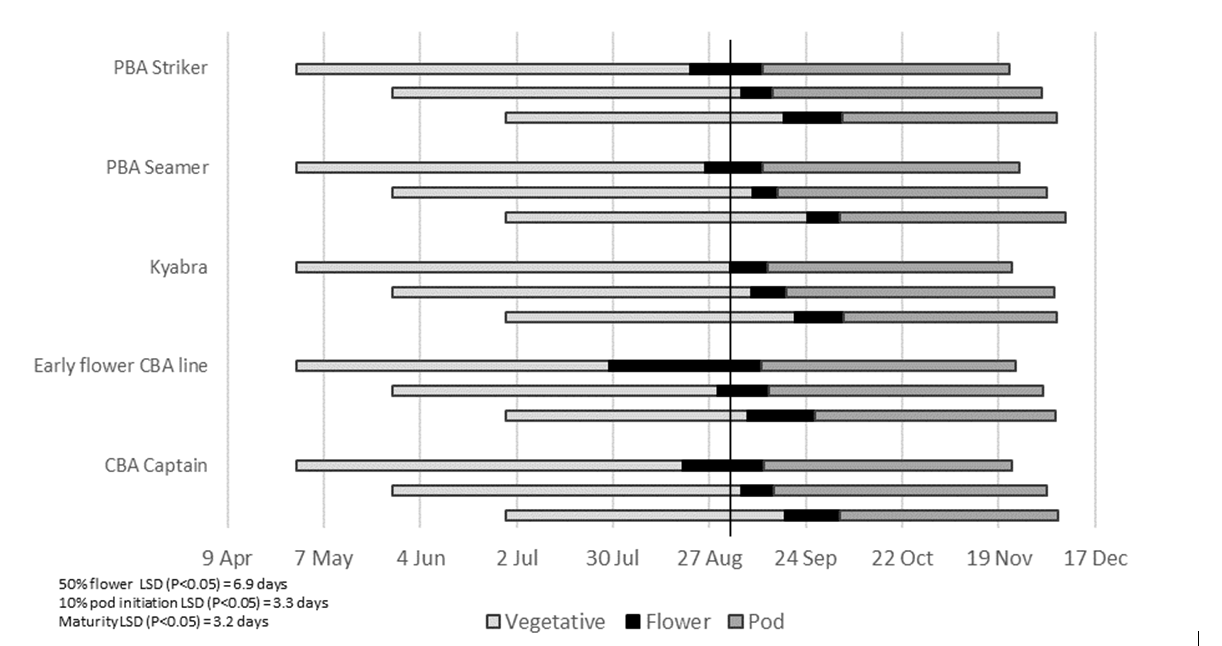
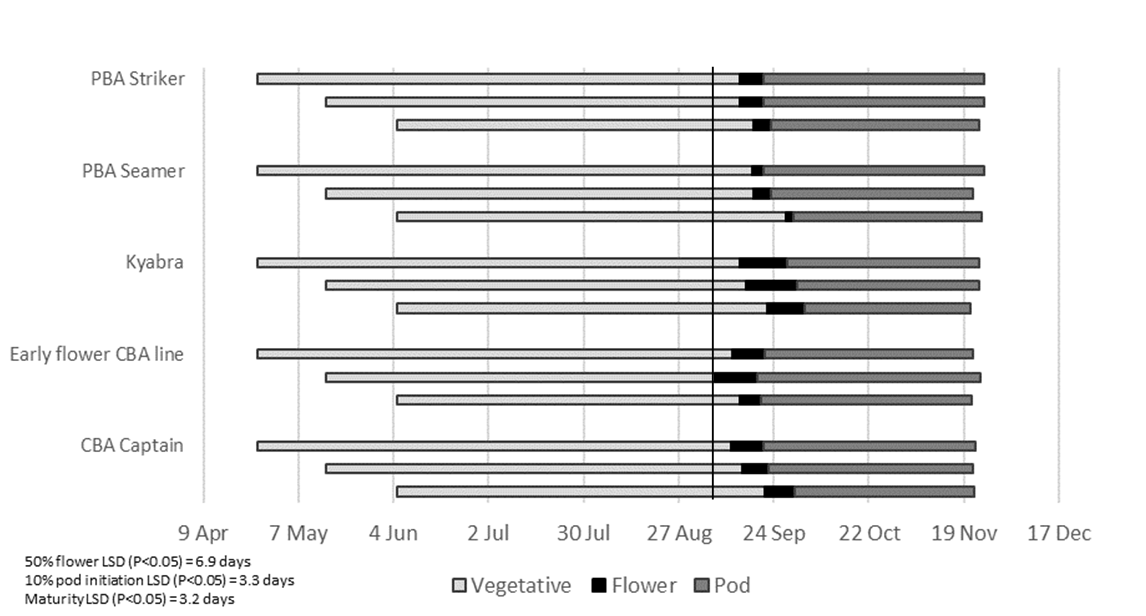
In contrast to the Tamworth findings, flowering did not commence at Wagga Wagga until 6 September, the day the average air temperature rose above 15°C for the first time (Figure 2). The first line to flower from the main sowing in mid-May was the early flower CBA line. Time to flowering was condensed over the three sowing dates (Figure 2 and Figure 3). Date of pod initiation at Wagga Wagga was also condensed, with a small but significant delay in pod initiation for Kyabra when compared to the early flower CBA line at each of the sowings (Figure 2).
Pod initiation, averaged over all genotypes at Tamworth for late April, late May and late June sowing dates, respectively, occurred 10, 13 and 32 days after average air temperature became optimal (3 September) (Figure 1). The delay in pod initiation, for the late June sowing at Tamworth, reflected the dry spring and depleted soil moisture in dryland cropping systems in northern NSW in 2020.
In contrast, when averaged over all genotypes, at Wagga Wagga, pod initiation for the late April, mid-May and early June sowings, respectively occurred 16, 18 and 25 days, after optimal average air temperature was achieved on 6 September, (Figure 2). Pod initiation at Wagga Wagga was in part delayed due to the higher level of temperature fluctuation in September, when temperatures rose and fell, above and below the optimal average air temperature of 15°C.
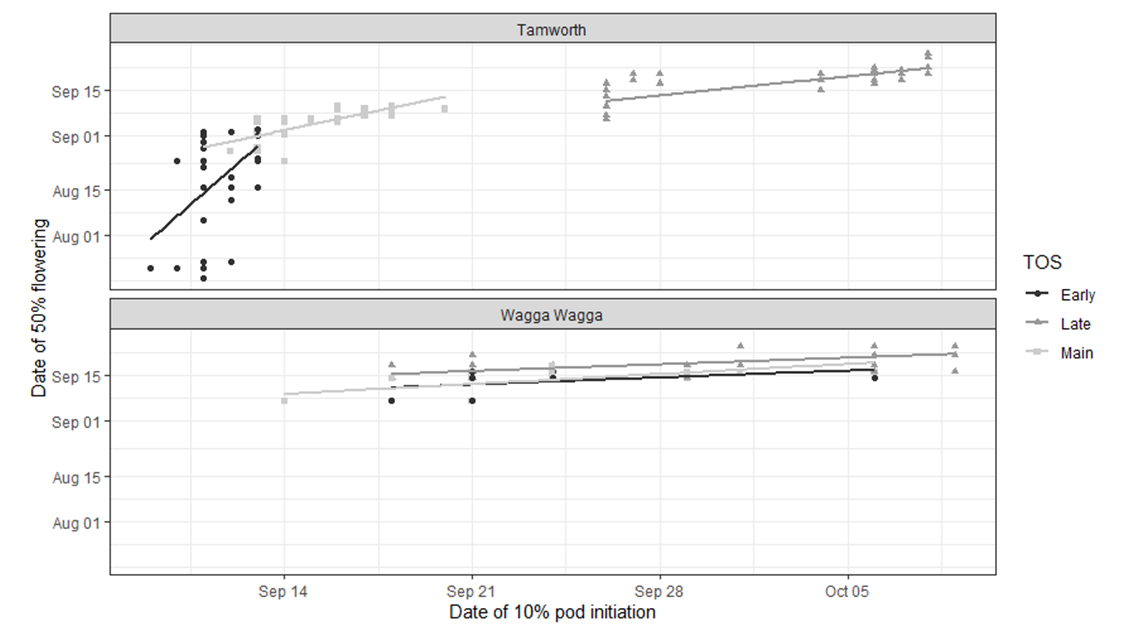
The relationships between date of 50 % flowering and date of pod initiation varied between the two sites (Figure 3). Genotypes sown early at Tamworth, had a spread of dates for the time to 50% flowering between 19 July to 9 September (52 days), while pod initiation occurred quickly, from 9 September to 13 September (4 days) (Figure 3). In comparison, the second and third sowing dates both had a variation in the time to 50% flowering of 17 days and 20 days, while pod initiation was spread over 9 days and 12 days, respectively (Figure 3).
The relationships between date to 50 % flowering and date of pod initiation, were compressed at Wagga Wagga when compared to Tamworth (Figure 3). There was overlap of both the date to 50% flower and the date of pod initiation between the three sowing times (Figure 3). Early and main sowing had similar dates to 50 % flowering from 7 September to 18 September and pod initiation from 14 September to 6 October. It is surmised, that this compression of dates for these phenological stages, may have been due to the greater variation in spring temperatures observed at Wagga Wagga, when compared to Tamworth.
Indictive yield response
When pooled across all genotypes, highest grain yield was observed when crops were sown between mid to late May, yielding 2.5 t/ha at Tamworth and 2.7 t/ha at Wagga Wagga (Figure 4). Sowing earlier than mid-May caused a yield loss of approximately 7 % at Tamworth and 18 % at Wagga Wagga (Figure 4). In part, the lower grain yield associated with early sowing, may be due to the longer time spent during the reproductive period in suboptimal temperatures.
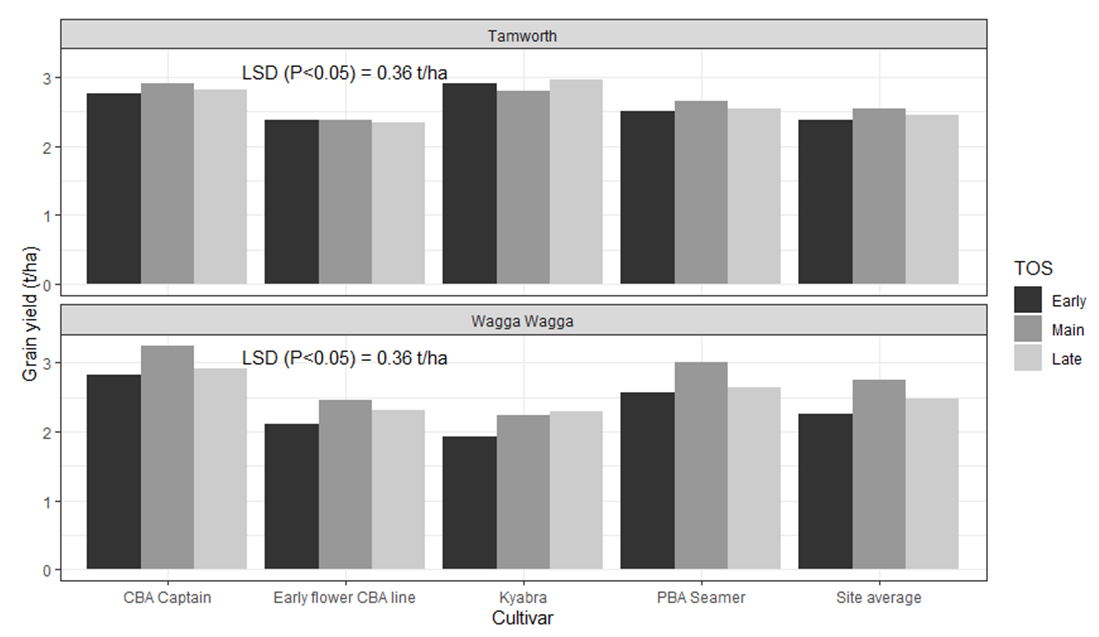
Individual varieties showed different patterns of yield rankings over the three sowing dates at both Wagga Wagga and Tamworth. Differences in yields between the three sowing dates for four genotypes, CBA Captain, early flower CBA line, Kyabra and PBA Seamer, at Wagga Wagga were significant, whilst at Tamworth they were not. When each of the four genotypes were sown early at Wagga Wagga there was a reduction in yield of between 12 % to 14 %, when compared to their main season sowing yields (Figure 4).
This loss in grain yield when crops were sown earlier than optimal can be partially explained by plants developing flowers that do not form pods or pods with seeds when temperatures are suboptimal. Current research, within this project, aims to look at the level of lost flowering opportunities and potential pods and seeds, when crops flower under suboptimal conditions, through measuring the conversion of reproductive nodes into pods and pods that contain seeds.
Summary
Matching developmental phases, in particular flowering and pod initiation to optimal environmental conditions increases the yield potential of chickpea cultivars. Importantly, results from these experiments show that early flowering does not translate to earlier pod initiation. This was particularly highlighted when comparing genotypes from an early sowing date at Tamworth in 2020.
The results from these experiments do however indicate that there is greater variation in pod initiation between genotypes from main and late season sowings, with earlier flowering genotypes initiating pods earlier than later flowering lines.
It can be seen from these experiments that early flowering does not equal early pod initiation or increased grain yield.
References
Berger JD, Buck RP, Henzell JM and Turner NC (2005) Evolution in the genus Cicer – vernalization response and low temperature pod set in chickpea (C. arietinum L.) and its annual wild relatives. Australian Journal of Agricultural Research 56, 1191-1200.
Clarke HJ and Siddique KHM (1998) Growth and Development. In ‘The Chickpea Book: A technical Guide to Chickpea production’ (Eds SP Loss, N Brandon, KHM Siddique) pp. 3-10. (Bulletin 1326 Department of Agriculture and Food, Western Australia: Perth).
Siddique KHM and Sedgley RH (1986) Chickpea (Cicer arietinum L.), a potential grain legume for south-western Australia: seasonal growth and yield. Australian Journal of Agricultural Research 37, 245-261.
Srinivasan A, Saxena NP and Johansen C (1999) Cold tolerance during early reproductive growth of chickpea (Cicer arietinum L.): genetic variation in gamete development and function. Field Crops Research 60, 209-222.
Yield Gap Australia (2018) (https://yieldgapaustralia.com.au), accessed 5 January, 2022)
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
The author would like to thank current and past staff that have worked on this project: Annie Warren and Helene Davidson at Tamworth and Rosy Raman, Muhuddin Anwar, Mark Richards, Jess Simpson, Asad Asaduzzaman and Joe Moore at Wagga Wagga.
The author would like to acknowledge the assistance of other research teams at both TAI and WWAI for trial management these include CBA lead Kirsty Hobson at TAI, including Judy Duncan, Andrew George, Ben Frazer, Belinda Rowe, Mandy Rowe and Madonna Rowe, winter cereal team lead at TAI by Rick Graham including Stephen Morphett, Peter Formann Rod Bambach, Bailey Skewes and Mick Dal Santo and pulse evaluation team at WWAI lead by Mark Richards including Michael McCaig, Ollie Owens and Karl Moore.
The author would also like to thank the farm managers: Scott Goodwin at Liverpool Plains Research Station, Breeza, Ross Kamphorst at Glen Innes Advisory and Research Station, and the farm managers and staff at Tamworth Agricultural Institute and Wagga Wagga Agricultural Institute.
Contact details
Dr Neroli Graham
NSW Department of Primary Industries
4 Marsden Park Road, Calala, NSW, 2340
Ph: 02 6763 1274
Email: neroli.graham@dpi.nsw.gov.au
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
GRDC Project Code: DAN1803-009BLX,
