Net Form Net Blotch management in barley
Net Form Net Blotch management in barley
Author: Lislé Snyman (Dept. Agriculture and Fisheries Queensland) | Date: 02 Mar 2022
Take home messages
- High levels of net form net blotch (NFNB) infection were observed in 2021 barley crops
- Continuous barley cropping increases the risk of stubble-borne diseases such as net blotch
- The NFNB pathogen is both seed and stubble-borne
- Virulence’s are dynamic and fluctuate in response to available host genotypes
- Management strategies for foliar diseases include resistant varieties, crop rotation, seed treatment, regular crop monitoring and timely fungicide application
- Resistance to fungicides have been reported in both NFNB and SFNB in Australia
- Limit fungicide application by spraying only when necessary, rotate fungicides with different modes of action and use recommended rates.
Background – Net form net blotch
Net blotch in barley is caused by one of two forms of Pyrenophora teres (P. teres). Net form net blotch (NFNB) is caused by P. teres f. teres (Ptt) and spot form net blotch (SFNB) is caused by P. teres f. maculata (Ptm). The two forms are morphologically identical and can only be distinguished by symptoms and molecular characterisation.
Symptoms of the net blotches are initially very similar, looking like small dark spots. They then develop into lesions with varying amounts of necrosis and chlorosis, determined by climatic conditions and resistance/susceptibility of the host. NFNB are characterised by net like dark brown necrotic lesions, whereas SFNB symptoms are characterised by dark circular or elliptic brown spots surrounded by a yellow chlorotic area.
Both forms are stubble-borne and survive from one season to the next on crop stubble or residue. The pathogen can also infect and survive on other cereals such as wheat and oats and can infect a wide range of other grasses (Agropyron, Bromus, etc). These are however regarded as minor hosts.
The net form net blotch pathogen is diverse, ever-changing and able to overcome the resistance in barley varieties. Virulence changes result from increased selection pressure on the pathogen by continuous barley cropping, no-till farming practices and widespread cultivation of genetically homogeneous crops.
Environmental conditions play a major role in the development of NFNB. Disease development and infection is favoured by frequent wet periods and mild temperatures.
Variation in the virulence of the pathogen is studied using differential sets. These sets include a number of lines or varieties with known resistances. For the set to be of local benefit, the inclusion of regional varieties is required and in order to identify changes in virulence, needs to be updated to include genotypes representing new sources of resistance.
Plant pathologists and breeders can use the knowledge on virulence in the pathogen population to identify and deploy sources of resistance effective against local pathotypes. Pathotype studies in Australia identified populations of Ptt to be quite unique to each state and reflect the cultivation of locally adapted varieties.
Net form net blotch in 2021
Net form net blotch occurs regularly in the northern region and samples are collected from crops throughout the season. In 2021, 14 samples of NFNB were collected from Qld barley crops. These were mostly collected from varieties Commander, Spartacus and RGT Planet with very high levels of disease observed in some crops.
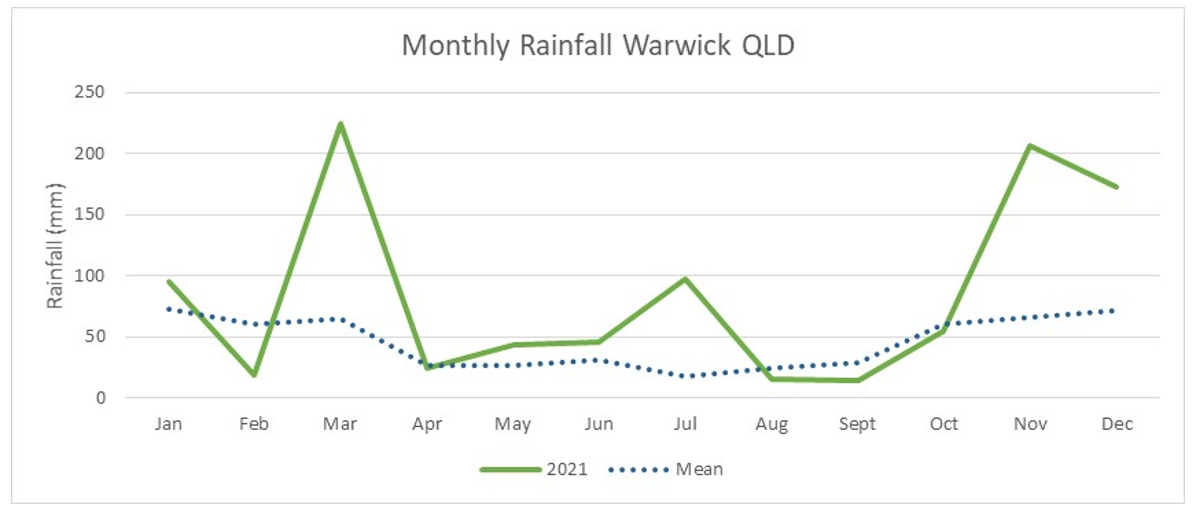
Despite above average rainfall in the 2021 season, disease incidence was not as prolific as anticipated. The wet spell in July delayed planting of many crops. The dry spell in August and September most likely restricted disease onset and progress in late planted crops (Fig. 1). However, some crops were severely impacted by high disease levels, particularly in varieties mentioned above and in barley-on-barley crops.
Figure 1. Monthly rainfall for the Hermitage Research Station, Warwick.
Disease management
Barley foliar pathogens are a significant challenge to the grains industry and a major constraint to profitable barley production, affecting both yield and quality. Many of these pathogens are genetically and pathogenically diverse, able to reproduce sexually and can rapidly develop new virulence’s and overcome genetic resistance.
The adoption of stubble retention practices has led to an increase in the incidence of stubble-borne diseases such as the net-blotches. Planting successive barley crops in the same paddock increases pathogen incidence.
Growing a high yielding, well adapted, resistant variety provides the most economic and environmentally friendly means of disease control. Genetic resistances need to be durable to provide long-term protection. Net form net blotch is best controlled by sowing varieties rated MS or better in combination with cultural practices that reduce inoculum load.
All current barley varieties and varieties considered for release are rated for resistance to a suite of diseases and pathogens through the National Variety Trial disease screening process. They are categorised in 9 resistance categories rating from resistant (R) to very susceptible (VS). These genotypes are screened annually in nationwide disease nurseries, with disease ratings assigned and reviewed on a yearly basis. The most up to date information on resistance ratings are available on the NVT website (https://nvt.grdc.com.au/nvt-disease-ratings).
The NFNB pathogen persists on plant residue. Cultivation of the same variety will lead to an increase in the presence of pathotypes virulent on that particular variety and put increased pressure on effective resistance genes. Best practice includes crop rotation with non-host crops such as wheat, canola and chickpea. NFNB is also seed-borne and can spread with infected seed. Various seed treatment products are registered for NFNB control. Systemic fungicides applied as a seed-treatment will provide protection against seed-borne diseases.
In susceptible varieties where yield potential is high, fungicidal control can be justified. Foliar fungicides should be aimed at protecting the key leaf solar panels present during grain filling – namely; the flag leaf sheath, the flag leaf (f), flag -1 (f-1), and f-2.
Resistance to Group 7 (SDHI) and Group 3 (DMI) fungicides has been identified in NFNB populations in SA and WA, respectively, with reduced sensitivity identified to other Group 3 fungicides in WA and Vic and both Groups 3 and 7 in SA.
To ensure that fungicides remain effective, it is important to limit fungicide application by spraying only when necessary, rotate fungicides with different modes of action and use fungicides at recommended rates. Avoid using tebuconazole as a stand-alone product in barley to avoid indirect fungicide resistance selection. By applying it for powdery mildew control, you can indirectly select for NFNB or SFNB isolates resistant to tebuconazole without the intention of controlling those diseases. Isolates resistant to fungicides can be spread through infected seed. It is beneficial to all to ensure that we use fungicides in such a way that we protect their longevity.
Fungicide applications are more effective if applied before disease becomes established in the crop. This requires regular monitoring to ensure crops can be sprayed at the first sign of disease. When conditions are favourable for disease development, more frequent crop inspections will be needed and repeat fungicide applications may be necessary.
Conclusion and 2022 planning
The absence and/or low incidence of many diseases in 2021 in the northern region does not mean that we can get complacent. With favourable environmental conditions, pathogens will continue to cause yield and quality loss and we have to make the right decisions to ensure that we can stay ahead of disease development and the evolution of the pathogen.
Continuous monitoring of the NFNB pathogen populations provides information on the virulence’s in Australia and aid in the identification of effective resistance for use in the development of resistant varieties.
References
Martin A, Poudel, B, Dahanayaka B, McLean M, Snyman L & Lopez-Ruiz F, 2021. Advances in understanding the epidemiology, molecular biology and control of net blotch and the net blotch barley interaction. In: Achieving durable disease resistance in cereals. Ed. R Oliver.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
Contact details
Lislé Snyman
DAF QLD
Hermitage Research Facility, 604 Yangan Rd, Warwick, Qld
Ph: 0428 324 932
Email: lisle.snyman@daf.qld.gov.au
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
GRDC Project Code: UOA2003-008RTX, DAQ2106-007RTX,