Dealing with herbicide resistance – what to know in 2022
Dealing with herbicide resistance – what to know in 2022
Author: Peter Boutsalis (School of Agriculture, Food and Wine, University of Adelaide, Plant Science Consulting P/L), Sam Kleemann (Plant Science Consulting P/L), Christopher Preston (School of Agriculture, Food and Wine, University of Adelaide) | Date: 21 Jul 2022
Take home messages
- Random weed surveys have been used since 2005 to identify the incidence of herbicide resistance across Victoria.
- Resistance testing can be used to identify effective herbicides.
- Using herbicides ‘smarter’ can greatly improve their effectiveness.
- Glyphosate should be used strategically, even if glyphosate resistance is present to protect against paraquat resistance.
- Clethodim + glyphosate mixtures can be synergistic, whereas clethodim + atrazine mixtures are antagonistic.
Ryegrass herbicide resistance in Victoria
Resistance to post-emergent herbicides
The GRDC has invested in the University of Adelaide since 2005 to conduct annual weed surveys across Victoria. The methodology involves randomly selecting 150–200 paddocks and collecting weed seeds of different species prior to harvest. Reasons for the presence of plants at the end of the season could include:
- resistant plants that have survived herbicides
- germination occurred post-herbicide application
- sub-lethal effects due to shading or poor herbicide/adjuvant choice/poor application equipment
- reduced herbicide uptake due to plant stress
- a combination of the points above.
Testing using pot trials under optimum spraying conditions (accurate herbicide application on non-stressed weeds at correct timings) is conducted the following season under outdoor winter conditions. These studies are aimed at identifying the incidence and changes in resistance mechanisms at 5-year intervals, in addition to monitoring for resistance to recently registered herbicides. The results are expressed as the percentage of paddocks containing resistant weeds as determined by a pot test and where survival in that pot test was ³20%. Seeds of target weed species ryegrass, brome, barley grass, wild oats, mustard, sowthistle and wild radish were sampled and later tested to several herbicides at recommended label rates.
Table 1: Resistance detected to key weed species from a random weed survey conducted in 2015 in western Victoria.
Species | Resistance detected in 2015 random weed survey | MoA Group |
|---|---|---|
Brome | 8% Fop and 10% sulfonylurea (SU) | 1 and 2 |
Barley Grass | 0% Fop and 7% SU | 1 and 2 |
Wild Oats | 10% Fop and 30% SU | 1 and 2 |
Mustard | 37% SU, 45% 2,4-D, 32% Atrazine, 47% Brodal® | 2, 4, 5 and 12 |
Sowthistle | 95% SU, 83% Imi | 2 |
Abbreviation: MoA, mode-of-action
Table 2: Percentage of paddocks containing herbicide resistant ryegrass in western Victoria to pre- and post-emergent herbicides. Paddocks were scored as resistant if the seeds collected exhibited ³20% survival. In the 2020 survey, Luximax® and Overwatch® were also included with no resistance detected.
Year | Hoegrass® | Axial® | Select® | Glean® | Intervix® | Glyphosate |
|---|---|---|---|---|---|---|
MoA | 1 | 1 | 1 | 2 | 2 | 9 |
2015 | 70 | 33 | 3 | 60 | 31 | 7 |
2020 | Testing underway | |||||
Year | Trifluralin | Rustler® | Avadex® | Arcade® | Boxer Gold® | Sakura® |
MoA | 3 | 3 | 15 | 15 | 15 | 15 |
2015 | 31 | 0 | 3 | 0 | 0 | |
2020 | 41 | 0 | - | - | 6 | 0 |
Abbreviation: MoA, mode-of-action
For each herbicide, recommended field rates plus recommended adjuvants were used.
Resistance was identified to grass selective herbicides in brome, barley grass and wild oats, but was found at much lower frequency relative to ryegrass (Table 1 and 2). Resistance in ryegrass was most prevalent for Hoegrass (70%) and Glean (60%), however many populations remain susceptible to other key selective herbicides, Axial and Select. Poor control with Select is often blamed on resistance, however these results clearly suggest that factors other than resistance (namely, cold conditions) are causing failures in the field. Fortunately, most ryegrass populations tested in the 2015 random survey remain susceptible to glyphosate (7% resistant). However, detection of glyphosate resistance is concerning because glyphosate is the most commonly used herbicide pre-sowing and for pre-harvest crop-topping. Studies to investigate the viability of glyphosate resistant ryegrass seed after crop-topping showed that, while the strategy was effective on susceptible seed set, it had no effect on sterilising resistant seed. Consequently, identifying glyphosate resistance early via testing can enable more appropriate product choice, and in circumstances where glyphosate resistance is prevalent, crop-topping in pulses with paraquat would be a more effective strategy.
Substantial resistance to trifluralin was also identified for ryegrass, increasing in incidence from 31% in 2015 to 41% in 2020. No resistance to Rustler (propyzamide), Overwatch or Luximax was detected from the random weed surveys indicating the resistance levels are low. Propyzamide has been used for over a decade with no cases of resistance reported in ryegrass.
Latest pre-emergent residual mode-of-action (MoA) herbicides
Several new pre-emergent herbicides with diverse mode-of-action have recently been released to the Australian market and provide an effective alternative to old chemistry. These include Mateno Complete (Group 12, 15 and unknown), Luximax (Group 30), Overwatch (Group 13), and Ultro (Group 23) which collectively have registration for use in a range of crops. Use of these herbicides, particularly in combination with older chemistry such as trifluralin or Avadex, can provide strong control of multiple resistant ryegrass and other important weed species. If used as part of an integrated weed control strategy, these alternative MoA herbicides are likely to reduce selection pressure on any one MoA.
Herbicide resistance testing
A weed control failure is not always due to herbicide resistance. By identifying which herbicides are effective and which are not, an optimised weed control strategy can be implemented. In Victoria in the past 5 years, resistance to the following species has been detected (Table 3).
Table 3: Summary of weed species tested for herbicide resistance from Victoria. Samples sent to Plant Science Consulting by growers or their agronomists between 2016 and 2022.
Species | Samples tested | Resistance detected | MoA Group |
|---|---|---|---|
Barley grass | 5 | None | - |
Brome | 5 | Fop, Dim | 1 |
Wild oats | 12 | Fop, Dim, Den | 1 |
Wild radish | 9 | Eclipse®, 2,4-D, Brodal | 2, 4 and 12 |
Ryegrass | 251 | Fop, Dim, Den, SU’s, IMI’s, trifluralin, glyphosate. None to Sakura, atrazine, paraquat. | 1, 2, 4 and 9 |
Abbreviation: MoA, mode-of-action
Resistance testing can be conducted on seeds collected prior to harvest or on living plants growing in the paddock. To illustrate the importance of resistance testing, two recent case studies are presented (Figures 1 and 2).

Figure 1. This grower had planned to use trifluralin in a wheat crop in 2022. Upon receiving the test that identified 95% resistance to trifluralin, another pre-emergent herbicide was chosen. If trifluralin had been used as initially planned, the outcome would have been a substantial yield penalty plus a sizeable increase in the ryegrass seedbank.
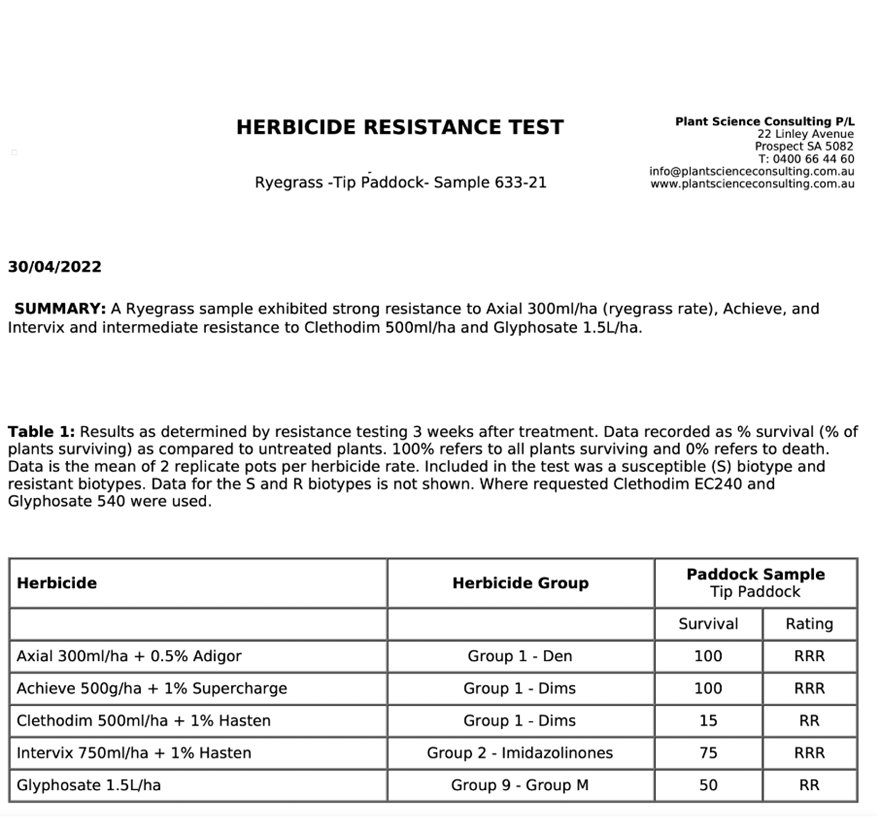
Figure 2. This grower used glyphosate as a knock-down and Achieve in a cereal crop in 2021 that resulted in a ryegrass blow-out causing a substantial yield penalty at a time when grain prices were high. With some forward planning, if seed or plants had been sent for testing in 2020, a more effective herbicide strategy could have been implemented delivering stronger weed management and a more profitable scenario.
Improving weed control
There are many factors other than resistance that influence herbicide performance and often involve application issues (for example, poor coverage, advanced and stressed weeds) and adverse environmental conditions. Resolving these issues can greatly improve control even if herbicide resistance is present. Resistance levels within individuals in a population can vary and, in many cases, these resistant plants can be controlled provided robust herbicide rates are applied at less advanced growth stages and under more optimum conditions. This is common in plants conferring weak resistance mechanisms to herbicides like clethodim and glyphosate, which can still be effectively controlled provided conditions are more optimal. In contrast, plants with strong resistance mechanisms, like target site modification with Group 1 and 2 herbicides, will be difficult to control irrespective of growth stage. In general, young plants possess thinner cuticles making herbicide entry easier. In addition, these plants are often also smaller allowing translocated herbicides (for example, clethodim and glyphosate) to more effectively reach the growing point (that is, the target site), enhancing control.
Using herbicides smarter
- Use high quality products and surfactants where recommended.
- Since paraquat does not translocate, coverage is more important than for glyphosate. Use nozzles that will ensure uniform coverage at water rates of at least 100L/ha on small seedlings and higher water rates on more advanced growth stages.
- For products that translocate, such as glyphosate and Group 1s, use coarser nozzles and lower water rates.
- Avoid combining too many active ingredients in the tank to reduce the likelihood of antagonism, particularly with low water volumes.
- Use ammonium sulfate to condition the water to improve efficacy, particularly if using hardwater (that is, high in calcium and magnesium). Ammonium sulfate needs to be added at the start and allowed to mix properly before adding other products. The concentration of ammonium sulphate required can vary depending on the water hardness (see useful resources at end of paper).
- Do not rely on a single mode-of-action along fence-lines, for example glyphosate. Resistance develops quicker along fence-lines due to the lack of crop competition and can move into the paddock via pollen or seed. Consider using residual herbicides in the autumn–winter.
- Consider using higher registered label rates if there is considerable shading and potential coverage issues.
- Maximise application by adhering to lower speeds and using the correct nozzles, pressure and boom height.
- Any combination of the above factors can reduce control thereby increasing the selection for resistance.
Best practice of using glyphosate, paraquat and clethodim
The double knock
A double knock approach involving glyphosate (to control the majority of susceptible individuals) followed with a robust rate of paraquat 1–5 days later is ideal (Figure 3). This approach reduces the selection pressure because the number of individuals exposed to paraquat is reduced since glyphosate does most of the ‘heavy lifting’. Even if glyphosate resistance is present in the paddock, a strategic double knock to reduce the population size exposed to paraquat is important to prevent paraquat resistance. Including other fast acting herbicides in combination with paraquat, such as the Group 14 herbicides Terrad’or® (700 g/kg tiafenacil) or Voraxor® (250 g/L saflufenacil + 125 g/L trifludimoxazin), can further reduce the selection pressure on paraquat. Tank-mixing glyphosate and paraquat is not recommended to avoid strong antagonism. A low number of paraquat resistant ryegrass cases have recently been confirmed in cropping paddocks in South-Western Victoria and South-Eastern SA. Some of these biotypes are also resistant to glyphosate.
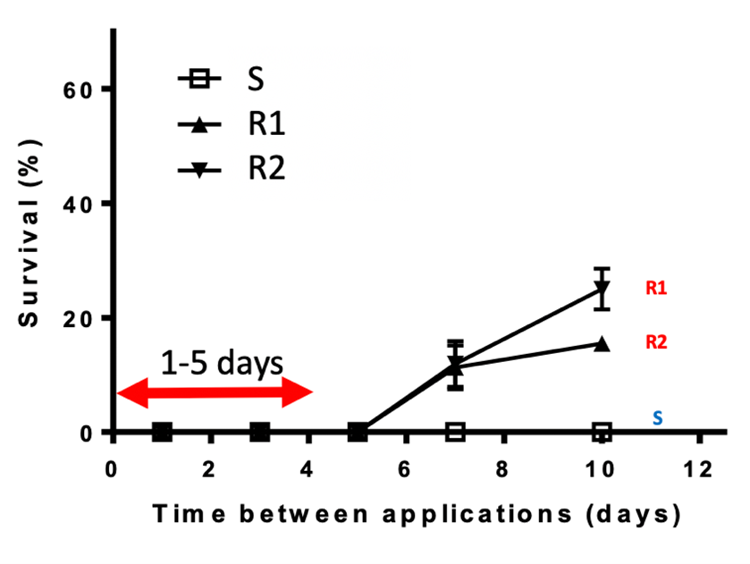
Figure 3. Double knock timing. Glyphosate applied to a susceptible (S) and two glyphosate resistant ryegrass biotypes (R1 and R2) followed by paraquat 1, 3, 5, 7 and 10 days after application. Trial work conducted by Dr Christopher Preston (The University of Adelaide).
Delayed ryegrass germination due to dormancy is becoming more problematic, allowing more ryegrass to evade knockdown control. However, pre-emergent herbicides with longer residual activity can assist and when used in combination with effective post-options, substantial control can be achieved. When resistance is prevalent to herbicides like glyphosate, the key is to identify alternative options to maximise control and prevent seedbank replenishment. Herbicide testing can easily identify the best herbicide options.
Clethodim + glyphosate tank-mix
All clethodim and certain glyphosate products are recommended for use in glyphosate-tolerant canola. Many populations of ryegrass with resistance to clethodim, glyphosate and both herbicides have been confirmed. In a recent pot study, ryegrass populations with resistance to clethodim, glyphosate and both herbicides were tested to a tank mix of 1.15L/Roundup Ready® PL and 500mL/ha Clethodim EC240. This combination was confirmed effective in most of the populations tested, with control averaging 95% compared to 73% and 79% for standalone glyphosate and clethodim, respectively (Figure 4).
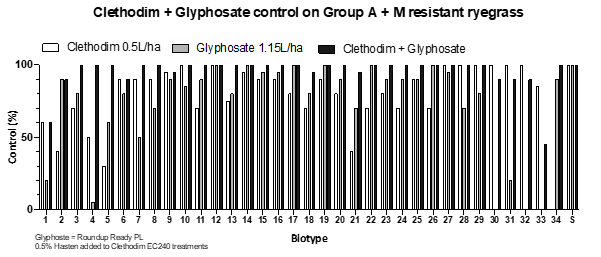
Figure 4. Control of DIM (Group 1, legacy A) and glyphosate (Group 9, legacy M) resistant ryegrass with tank-mixed clethodim and Roundup Ready PL herbicide.
Atrazine + clethodim tank-mix
The use of atrazine is an important tool as it controls many weed species including ryegrass. Resistance to atrazine in ryegrass is also rare meaning most populations remain susceptible to the herbicide. Tank-mix combinations of atrazine and clethodim have recently been shown to be antagonistic in controlling ryegrass. The recommendation therefore is to avoid tank-mixing to optimise the efficacy of both products (Table 4). Clethodim alone was shown to provide 10% greater control than clethodim + atrazine (tank-mix). Delaying the application between the two herbicides by up to 7 days provided the greatest improvement in control (30%) compared to the tank-mix.
Table 4: Increase in control (%) of treatments compared to a clethodim + atrazine tank-mix on 3-leaf ryegrass in outdoor pot studies. Data is the pooled response of a susceptible and three DIM resistant ryegrass biotypes. The trial was repeated with atrazine applied followed by clethodim treatments and vice versa (second trial). Rates are (1) clethodim EC240 @ 500mL/ha and atrazine WG900 @ 2kg/ha. Hasten and liquid AMS at 1% were included with each treatment. The clethodim + atrazine (tank-mix) treatment response was converted to 0 to calculate the improvement in control of clethodim and sequence treatments.
Treatments | Time after first herbicide | % Increase in control over Clethodim + Atrazine tank-mix |
|---|---|---|
Clethodim + Atrazine (tank-mix) | 0 | 0 |
Clethodim only | no atrazine | 10 |
Clethodim-Atrazine sequence | 10 min | 11 |
Clethodim-Atrazine sequence | 2 days | 17 |
Clethodim-Atrazine sequence | 7 days | 30 |
Impact of frost on clethodim efficacy
Certain herbicides, such as clethodim, are strongly affected by temperature. Reports of poor control in the field are common. An outdoor winter pot study was conducted to test the impact of frost on 250 and 500mL/ha Clethodim EC240 + 1% Hasten on FOP resistant ryegrass. Clethodim was applied to 2–4 tiller ryegrass growing in pots. Pots were transported to a location in Mt Torrens in the Adelaide Hills in July 2020 and exposed to the local frost conditions for three subsequent mornings (7am temperatures: -1oC (day 1), -1oC (day 2), +3oC (day 3). A proportion of the plants had been treated with clethodim prior to the frost treatment while others were treated on day 3 after the pots were returned to the Waite Campus. Clethodim was less effective on plants that were sprayed after the frost event (Figure 5). This finding supports trials conducted in 2012 and 2013 by the University of Adelaide (see WeedSmart factsheet ).
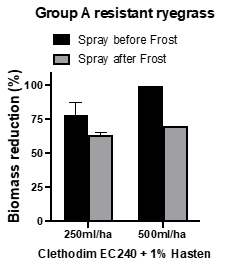
Figure 5. Effect of frost before and after application of clethodim on Group 1 (legacy A) FOP resistant ryegrass biotype 61.1-17.
Harvest weed seed control
The final opportunity to control weeds during the season is at harvest. Harvest weed seed control is an important strategy to destroy weed seeds that are remaining at the end of the season. Surviving plants can comprise of late germinators or resistant survivors that are likely to have accumulated resistance mechanisms and possess higher levels of resistance than the parent plants. Harvest weed seed control is a critical component to WeedSmart big 6 (herbicide and non-herbicide tactics) and should be implemented as a key herbicide resistance management strategy.
Conclusion
Several pre-emergent herbicide options remain to control multiple-resistant ryegrass as identified by recent national weed surveys. There are several factors that can contribute to poor weed control with resistance being only one of them. Optimising application equipment, timing, and understanding environmental factors that reduce herbicide efficacy are important. Glyphosate and paraquat can be used strategically even if resistance is present.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
Useful resources
Maximise clethodim performance: impact of frost
Contact details
Sam Kleemann
Plant Science Consulting P/L
Plant Science Consulting
Peter Boutsalis
University of Adelaide
Waite Campus, Glen Osmond SA 5064
peter.boutsalis@adelaide.edu.au
Plant Science Consulting P/L
Plant Science Consulting
@PBoutsalis
GRDC Project Code: UCS1306-001RMX, UCS2008-001RTX,
