Advances in the biological control of flaxleaf fleabane with a novel rust fungus
Advances in the biological control of flaxleaf fleabane with a novel rust fungus
Author: Ben Gooden (CSIRO) | Date: 22 Feb 2023
Take home message
The long-term aspiration of the biocontrol program is to reduce the growth and reproductive output of flaxleaf fleabane plants in marginal habitats, reduce invasion pressure on crop fields and in turn reduce the reliance on chemical herbicide application to control the weed, especially during fallow.
CSIRO’s research has shown the rust fungus to be a safe addition to the flaxleaf fleabane control ‘toolbox’, yet research into the efficacy of the biocontrol agent in a field setting has only just commenced. Future research is needed to optimise release methods and monitor the effects of fungal infection on weed populations over multiple growing seasons.
Introduction to the biological control of flaxleaf fleabane in Australia
Flaxleaf fleabane (Conyza bonariensis) is an annual herb, native to South America, that has become a significant agricultural weed of the grain growing regions of south-eastern Australia, where it greatly disrupts crop production (Wu 2007). This weed is a prolific seed producer that can spread long distances by both wind and water, thus necessitating an area wide approach to its management (Wu 2007). Example images of plant morphology and habitats prone to infestation are provided in Figure 1.
Flaxleaf fleabane affects crop production by greatly reducing stored water supplies in fallow, which affects subsequent crop emergence and growth. In recent years, the density and geographic extent of flaxleaf fleabane populations have expanded across south-eastern Australia, in part as a result of the adoption of minimum tillage practices that enhance seed survival and provide suitable conditions for germination and establishment. Populations of flaxleaf fleabane have also evolved resistance to some herbicides, making herbicide-resistant populations increasingly difficult to manage in many agricultural environments. Consequently, flaxleaf fleabane has become one of the most damaging summer fallow weeds in the northern grain region, with an estimated revenue loss in excess of $43 million per year for Australian grain producers (Llewellyn et al. 2016).
Flaxleaf fleabane infestations are considered by many grain growers to be particularly problematic in fallow, along roadsides, fencelines and irrigation embankments adjacent to crop fields. Flaxleaf fleabane populations are often able to proliferate in marginal habitats due to limited resources available for their control and challenges in coordinating control actions across various stakeholder groups that manage different land tenures in cropping regions. Uncontrolled flaxleaf fleabane populations in these marginal habitats in turn produce copious seeds that easily disperse to nearby crop fields, replenish the soil seed bank, and emerge in subsequent seasons. A key aim of management, therefore, is to reduce the reproductive viability of marginal flaxleaf fleabane populations and invasion pressure in adjacent crops.
Classical biological control (hereafter biocontrol) involves the introduction of a plant’s natural enemy (usually an herbivorous insect or fungal pathogen) sourced from its native range, with the aim of reducing the weed’s performance (usually a reduction in growth, competitive ability and/or reproductive output). Biocontrol represents a potentially valuable complementary control method for the management of flaxleaf fleabane given the success of previous biocontrol programs against other weeds in the Asteraceae family, such as parthenium (Parthenium hysterophorus) and skeleton weed (Chondrilla juncea). Indeed, the biocontrol of skeleton weed is deemed one of the most successful broadscale weed biocontrol programs in Australia (Ward 2014). By the 1950s, skeleton weed was considered one of the most destructive weeds in Australian productive systems. In the 1960s, CSIRO established the CSIRO Biological Control Unit in France (now the CSIRO European Laboratory), with the aim of identifying natural enemies of the weed that could potentially act as biocontrol agents in Australia. Subsequently, a rust fungus (Puccinia chondrillina) was identified and found to be highly host-specific to skeleton weed after rigorous risk assessment. The fungus was released into Australia in the 1970s (the first plant pathogen approved for deliberate introduction to Australia to help control a target weed) where it became widely established and dramatically reduced the population of the weed (by >80% in some areas) – benefits that have been sustained over the following five decades (Cullen 2012 and data provided to Ward 2014 by J Cullen).
The aims of the current CSIRO-led research into the biocontrol of flaxleaf fleabane were to:
(a) identify candidate biocontrol agents that attack flaxleaf fleabane in its native range
(b) undertake comprehensive host-specificity testing to demonstrate that they do not pose a threat to non-target plant species, and
(c) if approved by the authorities, release the biocontrol agents into Australia to help control the weed.
In this paper, CSIRO presents a summary of research that was undertaken to:
- select the microcyclic rust fungus Puccinia cnici-oleracei from Colombia (South America) as a candidate biocontrol for flaxleaf fleabane in Australia
- undertake host-specificity experiments to demonstrate the candidate agent’s safety for native and other important plant species in Australia, and
- undertake a small trial release of the rust fungus into the Australian environment in partnership with grain growers and other related stakeholders.
It should be noted that CSIRO has also identified and is currently undertaking host-specificity trials for several potential insect biocontrol agents on flaxleaf fleabane, including the stem-boring weevil Lixus caudiger identified in Brazil.
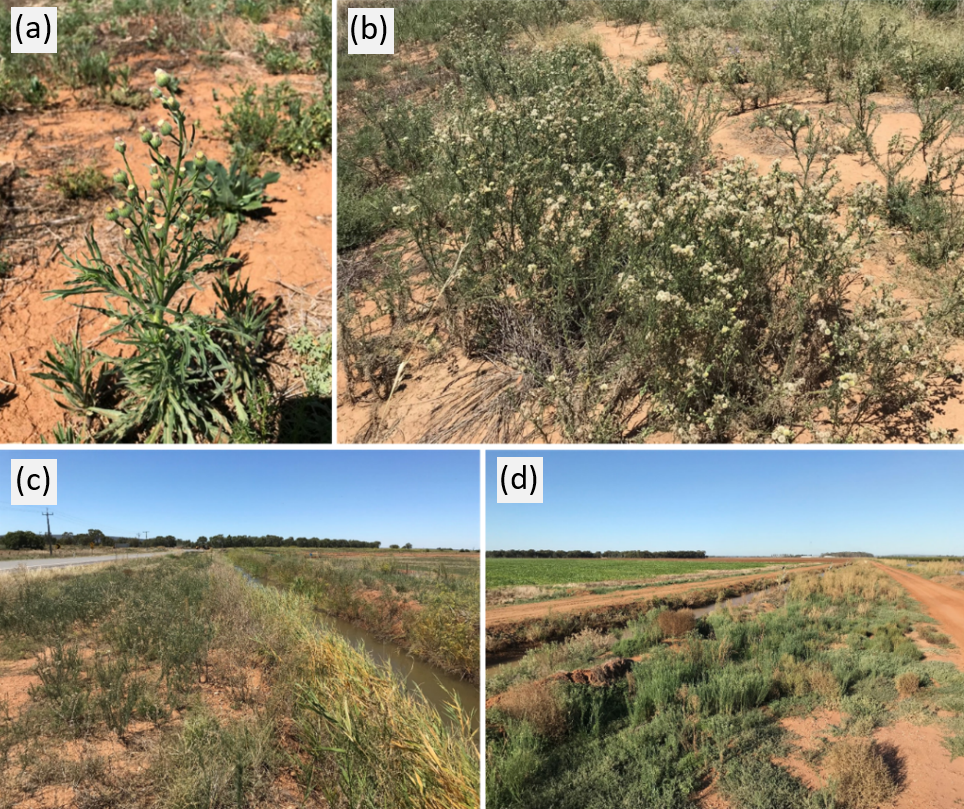
Figure 1. Example of (a) flaxleaf fleabane plant and (b) dense infestation in fallow; (c-d) examples of marginal habitats (e.g., roadsides, irrigation embankments, field margins, drainage lines) where residual flaxleaf fleabane populations are often not managed during the growing season and provide a seed source for re-invasion into adjacent crop fields.
Identification and risk assessment of the candidate biocontrol agent
In 2017, flaxleaf fleabane was endorsed by the Australian Government’s then Invasive Plants and Animals Committee (currently Environment and Invasives Committee) as a target for biological control. Subsequently, between November 2017 and May 2019, exploratory surveys for pathogens on Conyza species were performed in different regions of Colombia (South America), where pathogenic fungi had been previously recorded on Conyza. A microcyclic rust fungus, Puccinia cnici-oleracei, was identified during these native range surveys and prioritised as a candidate biocontrol agent for further host-specificity testing as described below (Morin et al. 2020).
The rust fungus infects young and old leaves, stems, and green flower parts of flaxleaf fleabane (Figure 2). The fungus obtains all its nutrients from flaxleaf fleabane by establishing intimate contact with the plant’s cells. Through continuous diversion of nutrients from the plant, the fungus reduces rates of photosynthesis, plant growth and reproduction but does not kill the plant altogether. Once a fungal spore germinates and penetrates the host plant leaf tissue, visible symptoms (yellowish speckling, followed by emergence of dark pustules where spores are produced) become evident after 2-4 weeks, after which time the infected leaves begin to die off (Figure 2). It is predicted that, if the fungus establishes widely and causes severe disease, it will decrease the reproductive output of flaxleaf fleabane populations and reduce the weed’s invasion potential in cropping areas.
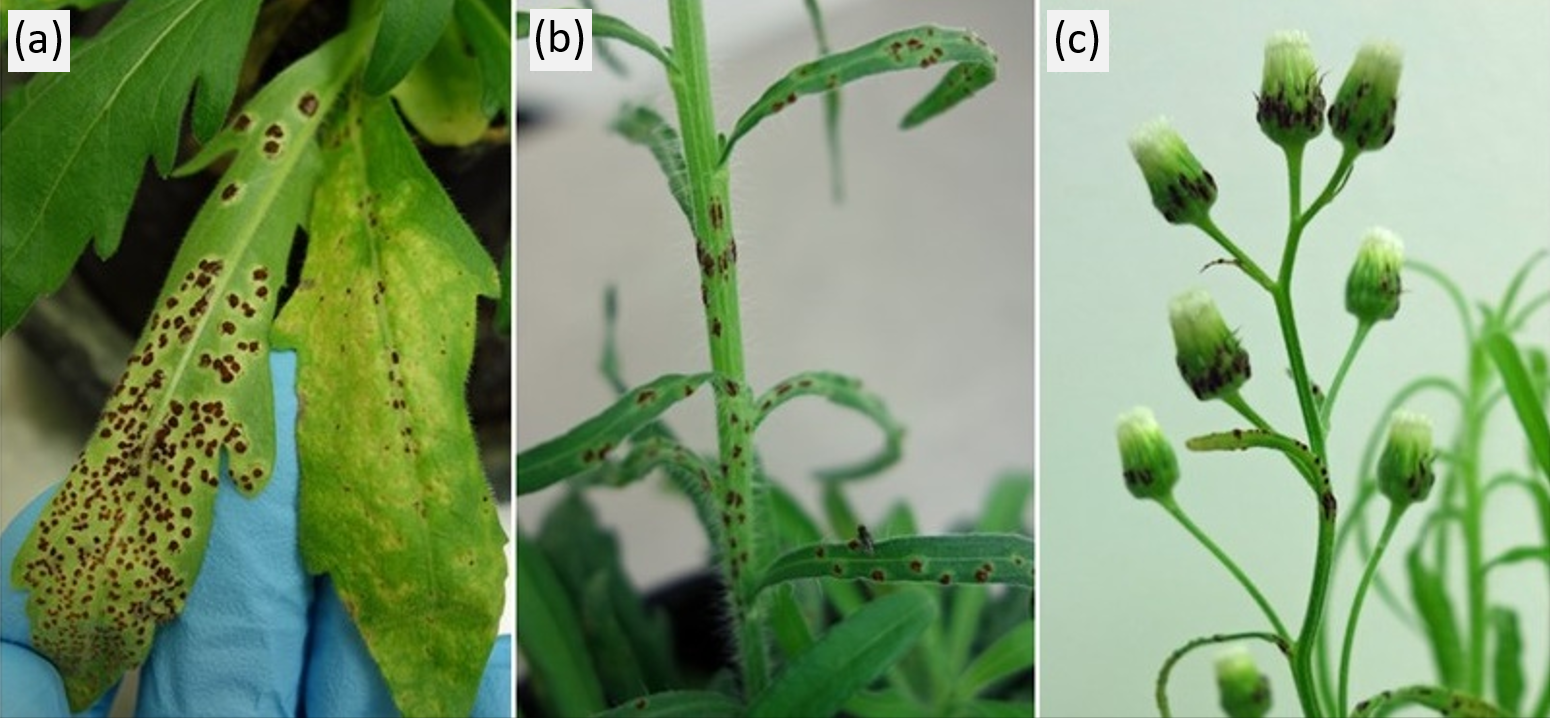
Figure 2. Characteristic disease symptoms caused by the flaxleaf fleabane biocontrol agent; a rust fungus named Puccinia cnici-oleracei. The fungus can infect leaves (a), stems (b) and flower heads (c). The dark brown pustules represent the reproductive stage of the fungal lifecycle, where spores are produced then released to infect nearby plants and gradually spread through the local flaxleaf fleabane population.
Candidate biocontrol agents are approved for release from quarantine into the Australian environment to help control a target weed only once rigorous host-specificity testing has been completed and the agent is shown to pose no risk of significant damage to non-target plant species. Such tests typically involve exposing a set of priority non-target plant species (including native Australian plants, ornamental and other important species) to the candidate agent under optimal conditions for growth and development, then assessing the level of damage to the plant and ability for the candidate agent to develop and complete its lifecycle.
In 2019, CSIRO commenced rigorous evaluation of the potential risks that the rust fungus could pose to non-target plant species in Australia (Morin et al. 2020). Research focused on species within the family Asteraceae that are most closely related to flaxleaf fleabane. This extensive host-specificity testing was performed in a quarantine facility and involved exposing flaxleaf fleabane and non-target plant species to the rust fungus under optimal conditions for infection. It was found that the fungus is highly host-specific to flaxleaf fleabane, is unable to complete its lifecycle on other plant species and poses no threat to the Australian environment (comprehensive results provided in Morin et al. 2020). Based on these research results and following a comprehensive risk assessment process and public consultation, the federal government regulators (then Department of Agriculture, Water and the Environment, DAWE) approved the release of the biocontrol agent into the Australian environment in June 2021.
An overview of current research on host-specificity trials for the stem-boring weevil Lixus caudiger can be found here.
Experimentally assessing the impacts of the biocontrol agent on flaxleaf fleabane
In December 2021, the fungus was experimentally released under field conditions on greenhouse-propagated flaxleaf fleabane seedlings. The aim of this experiment was to determine if flaxleaf fleabane plants could be successfully infected by the dried specimens of the lab-cultured rust fungus under variable light, humidity and temperature conditions in the Australian environment. The experiment was hosted outdoors at the CSIRO Black Mountain laboratories, as ongoing COVID-19 travel restrictions prevented us from undertaking the experiment in a crop setting.
The experiment consisted of planting multiple lab-germinated fleabane seedlings into a potting mix-sand propagation substrate within plastic tubs, exposed to the following treatments:
- Seedlings inoculated with the rust fungus, surrounded by a plastic bag to create a humid microclimate to stimulate sporulation;
- Seedlings inoculated with the fungus but without a plastic enclosure;
- Seedlings not inoculated with the fungus (control; note the control plants were not covered by the plastic bags).
Twelve replicate seedlings per treatment were used. The fungal spores were applied using a passive process by first hydrating dried infected leaves obtained from the lab culture for 1-2 hours in a water bath, mounting them onto bamboo stakes with the pustules facing downwards, then covering the bamboo stake and healthy plants with a plastic bag to maintain a warm and humid microclimate for at least 12 hours until the fungal spores had been released (Figure 3). The seedlings were then placed on a nursery bench outdoors under prevailing light, humidity and temperature conditions, to allow for development of infection symptoms within an environmental context. Seedlings were watered ad libitum to prevent dehydration in between rainfall events.
Post-inoculation monitoring consisted of randomly selecting 15 leaves per seedling and estimating the % cover per leaf showing symptoms of fungal infection. Approximately 2 weeks after the recipient plants were inoculated with the rust fungus, we detected characteristic signs of infection – i.e., light yellow-green speckles across the leaf surface (Figure 4). Strikingly, all 12 plants within the infected-covered treatment showed signs of infection. On average, 53 % of leaves were infected (presence/absence) and 18 % of the surface area of those leaves showed symptoms. However, only a single leaf on a single seedling in the infected-uncovered treatment had symptoms of infection, and none in the non-infected control seedlings. This provided evidence that maintaining a still, humid microclimate at the time of inoculation is critical for successful infection transfer of the fungus to flaxleaf fleabane seedlings under field conditions. Approximately 6 weeks post-infection, the inoculated seedlings that showed the early signs of infection (yellowish speckles) had developed dark brown lesions consistent with severe fungal infection and completion of the fungal lifecycle (Figure 4).
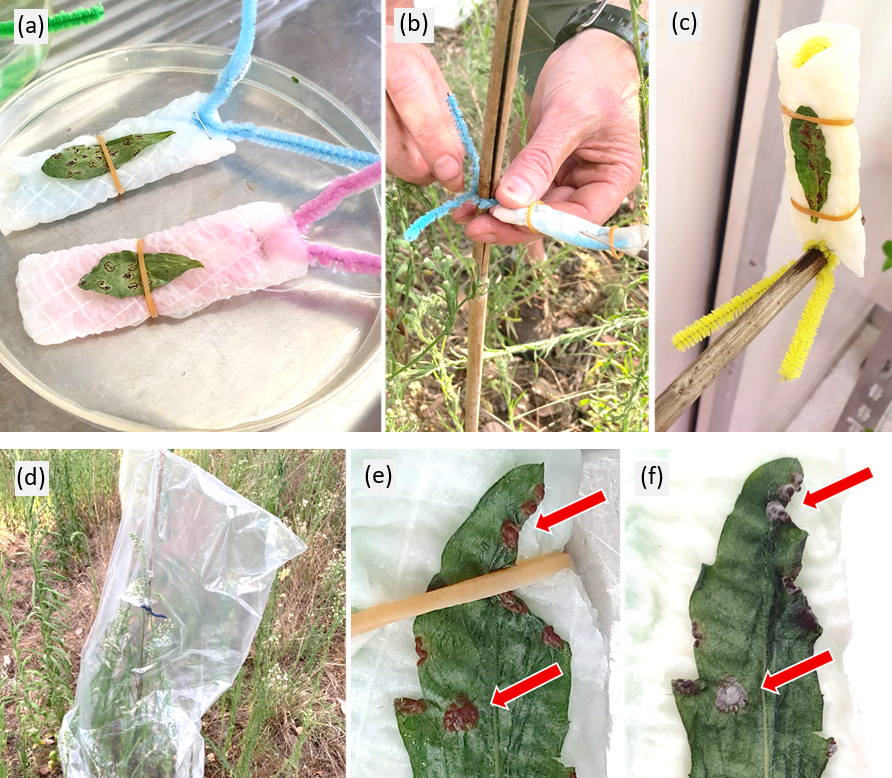
Figure 3. The process of setting up the biocontrol agent release. Dehydrated, infected leaves are (a) rehydrated in a water bath then (b-c) mounted and tied to a stake with rehydrated pustules facing downwards which is placed over a set of healthy flaxleaf fleabane plants and (d) covered with a plastic bag for at least 12 hours to maintain a humid and warm microclimate. Successful release of viable spores is indicated if the pustules turn from brown (e) to a fluffy grey colour (f).
Figure 4. Infection symptoms on flaxleaf fleabane plants experimentally inoculated with the rust fungus under field conditions.
Levels of infection, plant growth and reproductive output were monitored monthly to May 2022. Multiple infection events occurred between January and May 2022, whereby the first set of lesions that developed in January produced spores that spread to nearby healthy leaves that subsequently became infected, and so on. In this way, infection progressed over the entire body of each plant as they developed through to adulthood, with lesions eventually being detected on stems, inflorescences and flower heads. These observations confirmed that the fungus was able to readily infect flaxleaf fleabane plants growing outdoors over multiple months, under variable prevailing climate conditions.
At the conclusion of the experiment, plants were harvested, dried, and measured for height, biomass, number of inflorescences (flowering stems), capitulae (flower heads) and infection levels. There was no difference in the biomass of flaxleaf fleabane plants between the different inoculation treatments (data not presented). This may have arisen because the non-infected control seedlings eventually became infected with the fungus from spores spreading from the nearby infected plants. Future experiments would need to retain non-infected control plants for the duration of the experiment using fungal exclusion treatments to truly test the effects of infection on plant growth in the field.
A linear regression analysis revealed that reproductive output (measured as the number of flower heads per inflorescence, y-axis on Figure 5c) declined significantly with increasing percentage of leaves infected by the fungus (R2 = 0.15, F = 8.6328, P = 0.0050); note the contrasting condition of the inflorescence with severe infection by the fungus and distorted, stunted flower heads (Figure 5a) versus the healthy inflorescence not infected by the fungus (Figure 5b). On average, severely infected inflorescences produced 50-60% fewer flower and seed heads than non-infected inflorescences (Figure 5c). These results indicate that, under optimal conditions supporting high infection severity, the fungus can reduce the overall reproductive output of host plants. Reduced reproduction is likely a direct consequence of the fungus lowering the photosynthetic efficiency of infected leaves, in turn reducing the plant’s ability to assimilate available carbohydrates into inflorescence development and flower and seed production.
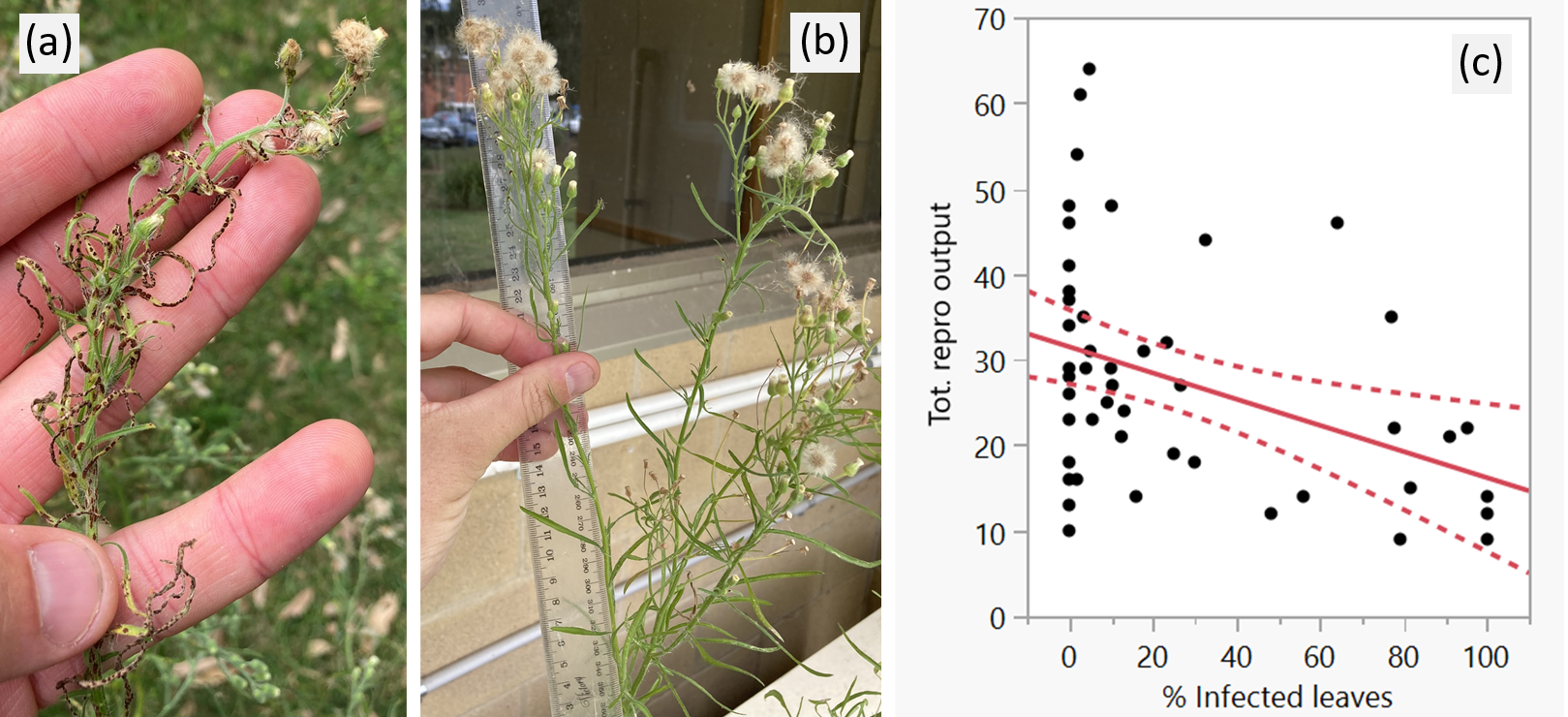
Figure 5. Results of fungal inoculation experiment on the reproductive output of host flaxleaf fleabane plants: (a) heavily infected and (b) non-infected inflorescences, and (c) linear relationship between reproductive output (number of flower heads per inflorescence) and % of infected leaves per inflorescence.
Pilot releases of the rust fungus throughout Australia with community stakeholders
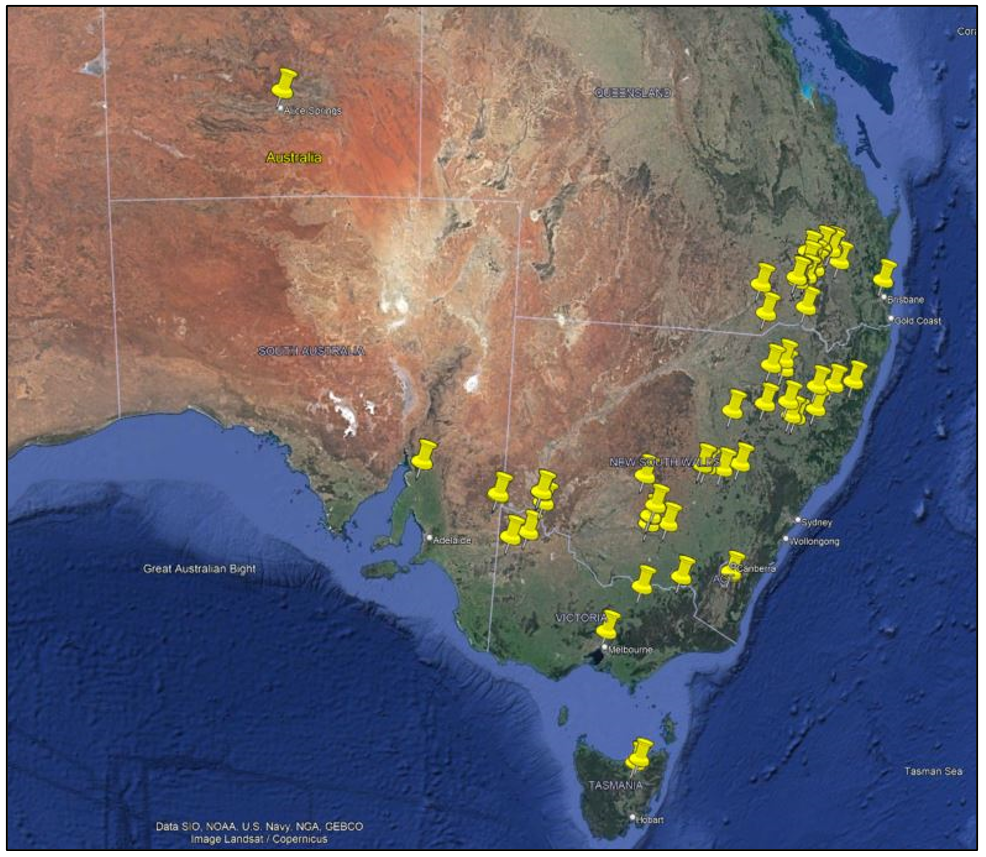
Commencing in September 2022, CSIRO launched a small pilot program in partnership with select landholders and other weed management stakeholders from the grain sector that aimed to trial release of the rust fungus on flaxleaf fleabane across south-eastern Australia using the bamboo stake-bag method described above. Interest from potential participants in the program was elicited through a joint GRDC-AgriFutures-CSIRO media campaign. 54 stakeholders were selected for participation in the program, comprising 39 private landholders/growers, 7 professional agronomists, 3 research institutes (University of Sydney, CSIRO, Northern Territory Arid Zone Research Institute), 2 biosecurity officers from local councils, 2 plantation industry groups and 1 Landcare network. Altogether, participants were sent 336 biocontrol agent release kits for dissemination in the field using the agreed release method. Releases were made nationwide, focussing on the south-eastern parts of Australia where flaxleaf fleabane infestations cause the greatest impacts on crop yield (25 releases in NSW, 16 QLD, 5 SA, 5 VIC, 2 TAS, 1 NT, Figure 6).
Releases of the rust fungus were made by registered participants between late September and mid-December 2022 during fair weather days, usually shortly after periods of rainfall under high humidity conditions, in marginal areas adjacent to crop fields (e.g., fencelines, roadsides, drainage ditches, field in fallow) with a dense foliage coverage of flaxleaf fleabane. Participants were sent release kits containing dried flaxleaf fleabane leaves infected with the rust fungus and comprehensive instructions on how best to release the fungus to maximise likelihood of infection of recipient flaxleaf fleabane host plants. In March 2023, CSIRO will work with stakeholders to monitor for signs of fungal infection at the release sites and quantify overall rates of infection and identify regions and habitat conditions under which infection is most likely to occur. The results of this small pilot release program will improve the efficiency by which the biocontrol agent is released in future as part of a potential larger scale, nationwide mass-release program across Australia.
Figure 6. Distribution of biocontrol agent release sites across south-eastern Australia.
Management implications and future research aspirations
The long-term aspiration of the biocontrol program is to reduce the reproductive viability of flaxleaf fleabane plants in marginal habitats, invasion pressure on crop fields and in turn reduce the reliance on chemical herbicide application to control the weed, especially during fallow. CSIRO’s research conducted over the past several years has shown the rust fungus to be a safe addition to the flaxleaf fleabane control ‘toolbox’, yet research into the efficacy of the biocontrol agent in a field setting has only just commenced, with future research needed to optimise release methods and monitor the effects of fungal infection on host plants over multiple growing seasons.
It is predicted that, even where the rust fungus establishes successfully in the field, infection is unlikely to result in significant reductions in the population size of flaxleaf fleabane for several years. As such, biocontrol represents a longer term and self-sustaining means of gradually reducing weed invasion pressure across productive landscapes. It is expected that the fungus will spread from one plant to the next very slowly at first, but the rate of spread will likely accelerate once the overall abundance of the rust fungus builds up in the local flaxleaf fleabane population. Based on our knowledge of other successful biocontrol agents that have been released previously in Australia (e.g., skeleton weed, Ward 2014), broadscale spread of the fungus would be expected to take several years. Furthermore, the combination of several biocontrol agents may enable more robust control of target weeds. In this way, further research into the biocontrol of flaxleaf fleabane with insects may provide enhanced biocontrol solutions.
When considered in isolation, classical weed biological control is not a silver bullet and will not eliminate flaxleaf fleabane from an area altogether or replace the need for deployment of chemical and mechanical control methods. However, by reducing flaxleaf fleabane’s growth and seed set, biocontrol agents could slow the rate of weed spread both within and outside of cropping areas and hence reduce the frequency of re-infestation in fallow. Widespread establishment and spread of the rust fungus may gradually reduce the quantity of chemical herbicide required to suppress flaxleaf fleabane populations and may thus be especially valuable in areas where the weed has developed herbicide resistance. Future research would be required to develop methods of integrating the effects of the fungus (likely most active in marginal habitats comprising unmanaged flaxleaf fleabane populations) with intensive chemical and mechanical control methods deployed on flaxleaf fleabane infestation in fallow.
References
Cullen (2012) Chondrilla juncea L. – skeleton weed. M. Julien, R. McFadyen, J. Cullen (Eds.). Biological Control of Weeds in Australia, CSIRO Publishing, Collingwood (2012), pp. 499-509.
Llewellyn et al (2016) Impact of Weeds on Australian Grain Production. Report for GRDC. CSIRO, Australia.
Morin et al (2020) Information package to support the application to release the rust fungus Puccinia cnici-oleracei (ex. Conyza) for the biological control of flaxleaf fleabane (Conyza bonariensis) in Australia. CSIRO.
Ward (2014) Biological control of skeleton weed.
Wu (2007) The biology of Australian weeds 49. Conyza bonariensis (L.) Cronquist. Plant Protection Quarterly, 22(4), p.122.
Further information
Flaxleaf fleabane: a weed best management guide
Flaxleaf fleabane biological control. Developing complementary management options for a difficult to control agricultural weed.
Background on CSIRO weed biological control.
New fungus to help landholders fight fast spreading weed. Groundcover August 2022.
Acknowledgements
This research is part of the project ‘Underpinning agricultural productivity and biosecurity by weed biological control’, led by AgriFutures Australia and funded by the Australian Government Department of Agriculture, Fisheries and Forestry as part of its Rural R&D for Profit program (PRJ-012377), with co-investment from CSIRO, GRDC and NSW Biocontrol Taskforce. The CSIRO component of research is led by Dr Raghu Sathyamurthy (Research Director), supported by Dr Michelle Rafter and Dr Ben Gooden.
Exploratory surveys of the rust fungus in Colombia, South America, were coordinated by Dr Louise Morin (CSIRO), with assistance from collaborators based at the Universidad Nacional de Colombia Sede Medellín. Host-specificity testing of the rust fungus on native and other important plant species under quarantine conditions in Australia was delivered by CSIRO researchers Dr Gavin Hunter, Dr Kylie Ireland, Caroline Delaisse, and Isabel Zeil-Rolfe.
Development of fungus release methods and implementation of the small pilot community-led release program were undertaken by CSIRO researchers Dr Lauren Kaye, Caroline Delaisse and John Lester under direction of Dr Ben Gooden. We thank the many growers and weed management stakeholders throughout Australia for their generous support in testing the fungus during spring 2022.
Research into the biological control of flaxleaf fleabane with insects is being delivered by Dr Vincent Lesieur, Thierry Thomann, Mireille Jourdan, Dr Michelle Rafter and Dr Kumaran Nagalingam with assistance from collaborators based at the Universidade Regional de Blumenau (Brazil).
Contact details
Dr Ben Gooden
Senior Research Scientist
Health & Biosecurity CSIRO
GPO Box 1700 Canberra ACT 2601
Clunies Ross Street, Acton, ACT 2601
Ph: +61 2 6218 3896
Email: ben.gooden@csiro.au
Date published: February 2023
GRDC Project Code: RDC1607-003OPX,