Fungicide resistance in wheat powdery mildew
Fungicide resistance in wheat powdery mildew
Author: Fran Lopez-Ruiz (Curtin University), Kejal Dodhia (Curtin University), Steven Chang (Curtin University), Steven Simpfendorfer (NSW DPI), Sam Trengove (Trengove Consulting) | Date: 07 Feb 2023
Take home message
- The wheat powdery mildew pathogen has developed resistance to QoI (Group 11, strobilurin) and DMI (Group 3, triazole) fungicides
- Resistance to both fungicide groups have been found at high frequencies across wheat growing regions of NSW, Vic, Tas and SA
- Non-chemical disease management practices are key for managing wheat powdery mildew QoI and DMI resistance outbreaks, especially under conducive climatic conditions
- The use of more genetically resistant varieties in wheat powdery mildew-prone regions is essential to slow down the further development and spread of fungicide resistance.
Introduction
Blumeria graminis f. sp. tritici is the causal agent of wheat powdery mildew. In Australia, it has been associated with yield loses of up to 25% under conducive conditions due to a reduction of photosynthetic leaf area and nutrients available to the crop (Trengove et al., 2021; Dodhia et al., 2021). Wheat powdery mildew can be controlled with azole inhibitors (DMI, Group 3) and strobilurins (QoI, Group 11) in regions where resistance is not present or at low levels (Dodhia et al., 2021). The development of resistance not only threatens effective crop protection, but also reduces the number of modes of action (MoA’s), available to growers, contributing to the increase of fungicide resistance risk within those systems.
In Australia, over 10 Mha are sown with wheat annually, thus providing a large breeding ground for this pathogen (ABARES, 2020). Mildew infections are most common in areas with medium to high rainfall, as cool humid conditions are required for optimal disease development. Irrigated crops often provide the right conditions for powdery mildew development.
There are no current estimates of the cost of wheat powdery mildew to the Australian wheat industry. Analyses conducted in the 1990s and 2000s show that in the absence of control, losses of up to AU$18 million/year can be expected (Murray and Brennan, 2009).
The first report of strobilurin resistance in Australian wheat powdery mildew attributed to field failure observations, was from the states of Victoria and Tasmania in 2016 (Dodhia et al., 2021). QoI resistance is now accepted to be widespread across regions of New South Wales (first detection in 2015), South Australia (first detection in 2019), Victoria and Tasmania.
Although resistance to DMI fungicides was detected as early as 2015 in Tasmania and New South Wales, it was not until a few years later that it started to have a practical impact in the field. DMI resistance was later detected in Victoria (2018) and South Australia (2019).
In 2020, there were concerns across wheat-growing regions of New South Wales and northern Victoria on the performance of fungicides from the DMI group. Despite crops receiving 2-4 fungicide applications during the season, wheat powdery mildew remained a problem for growers in some areas (Simpfendorfer et al., 2021).
DMI fungicide resistance was detected at very high frequencies in samples collected from paddocks around Edgeroi, Wee Waa, Albury, Rennie, Balldale, Deniliquin, Jerilderie, Hillston and Yenda in NSW, and Cobram and Katamatite in Victoria. Genetic and phenotypic analyses of the isolates obtained from these locations revealed a combination of mutations in the DMI fungicide target gene that were associated with the resistance observed to some DMIs. Additionally, all samples tested had some level of strobilurin fungicide resistance.
This paper summarises the in-planta analyses conducted with these DMI resistant isolates. We believe that the information generated by this research can support the paddock management of wheat powdery mildew and fungicide choice in the affected regions. An updated map of the distribution of the resistance will be provided at the time of the presentation.
Methods
In-planta assays
Wheat plants from the susceptible variety Trojan were grown in 19 cm diameter pots in a glasshouse. Three pots containing five plants each were grown for each of the fungicide rates tested. At the four leaf stage, plants were transferred to a spray cabinet and each fungicide sprayed at the recommended speed and water volume to mimic field application, and with a droplet size similar to that used in the field. Plants were sprayed at 0.5, 1, and 2 times the lowest registered field rate for propiconazole 550 EC (70 mL/ha) and the maximum rate for Aviator® Xpro® (500 mL/ha). Control plants were sprayed with water. Plants were left to dry for 24h and then transferred back to the glasshouse where whole plant inoculation with spores of the wild type (non-resistant) and resistant B. graminis f.sp. tritici isolates was conducted. Sensitive and resistant isolates were bulked up in vitro in order to obtain spore volumes for inoculation of the wheat plants. Wheat powdery mildew symptoms were scored 14 days after inoculation. There were three replicates of each fungicide treatment.
Results and discussion
The experiments reported here mimicked field conditions to determine whether particular fungicides could be impacted in their performance when DMI-resistant genotypes of the wheat powdery mildew causal agent B. graminis f.sp. tritici are present in the field.
The visual inspection of wheat plants treated with propiconazole at 70 mL/ha (lowest label rate) and inoculated with wild type and DMI-resistant isolates, revealed important differences in symptom levels (Figure 1A and 1B). While plants inoculated with the wild type showed no powdery mildew symptoms after 14 days, no fungicide control was observed in the case of the plants inoculated with the DMI-resistant strain, which produced abundant lesions and high numbers of spores. When plants were sprayed with propiconazole at 140 mL/ha and then inoculated with the DMI-resistant isolate, a significant reduction of powdery mildew symptoms was observed (Figure 1C). However, symptoms were still clearly visible and lesions were still producing a high number of spores.
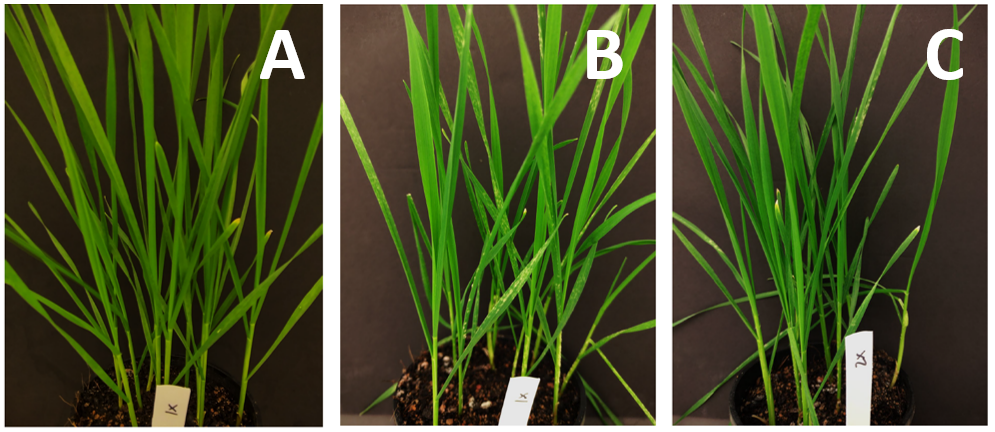
Figure 1. Wheat powdery mildew symptoms on wheat plants variety Trojan sprayed with different rates of propiconazole 550 EC and inoculated with wild type and DMI-resistant B. graminis f.sp. tritici isolates. (A) Propiconazole at 70 mL/ha and inoculated with wild type isolate; (B) Propiconazole at 70 mL/ha and inoculated with DMI-resistant isolate; (C) Propiconazole at 140 mL/ha and inoculated with DMI-resistant isolate.
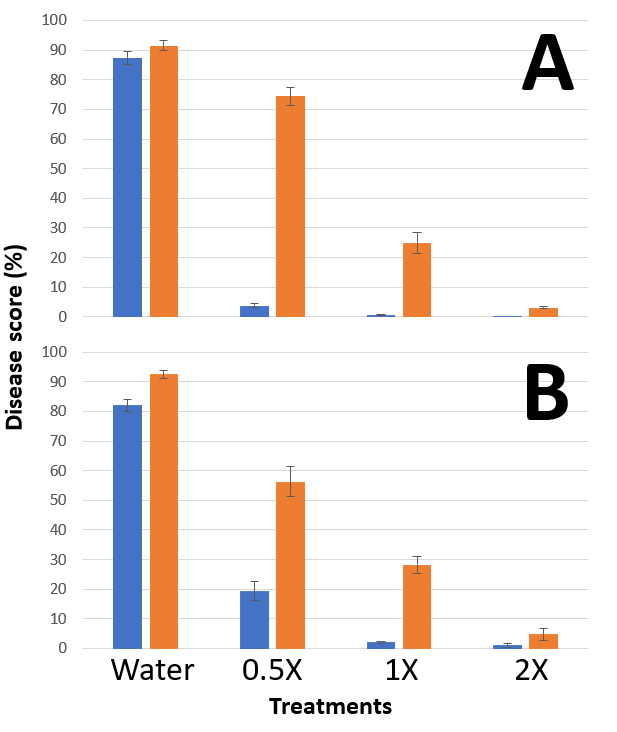
Treatment with propiconazole at 35 mL/ha (half the lowest label rate) reduced disease levels by more than 95% compared to the control, which provides an idea of the efficacy of this product when used against a wild type powdery mildew population under laboratory conditions (Figure 2A). This result does not support the use of rates outside of those specified on the registered label and is only presented here to provide a better understanding of the effects of mutations on fungicide performance.
It is also important to understand fungicides were applied under ideal conditions in this experiment that would rarely occur under field conditions. The lack of fungicide activity diminishing factors like the effect of UV light, rain and other factors such as microbial activity, means that the use of the presented fungicide rates under field conditions where DMI-resistance is high in frequency would probably lead to lower levels of control than recorded here. This could offer an explanation to the fact that some growers have experienced an important lack of field control of wheat powdery mildew when crops were sprayed with propiconazole at maximum label rates.
In addition to this, even when a fungicide can still provide control, the protection period often decreases and disease symptoms can revert to pre-spray levels in shorter than usual times in the presence of fungicide resistant pathogen populations.
Although succinate dehydrogenase inhibitor (SDHI) fungicides are not regarded as very good mildew control actives, some formulations can be used for the control of other diseases affecting wheat. We wanted to determine the capacity for the combination of the SDHI (Group 7) bixafen and the DMI prothioconazole (sold as Aviator® Xpro®) to reduce wheat powdery mildew levels produced by the DMI-resistant isolate.
Figure 2. Disease scores obtained for wheat plants of the powdery mildew susceptible variety Trojan sprayed with different rates of propiconazole 550 EC (A) and Aviator® Xpro® (B) and inoculated with wild type (blue) and DMI-resistant (orange) B. graminis f.sp. tritici isolates.
Disease levels observed for plants treated with Aviator® Xpro® at 250, 500 (maximum label rate) and 1000 mL/ha (2X) (Figure 2B), followed a similar pattern to that of propiconazole (Figure 2A) for both the wild type and the DMI-resistant isolates.
A typical fungicide resistance management recommendation is to use different fungicide MoA’s (Ireland et al., 2021). This is a logical approach to manage the problem, but does not necessarily provide control for all diseases in all situations if some MoA have poor inherent control activity against a particular pathogen. Despite the high activity exhibited by Aviator® Xpro® against other diseases, the relative lower effect of SDHI actives against wheat powdery mildew means that the DMI partner will be under higher selection pressure, especially when faced with wheat powdery mildew outbreaks in conducive environments.
In our experiments, it was demonstrated that propiconazole has a significantly lower activity against wheat powdery mildew DMI-resistant isolates. This has been further supported by the numerous field reports of lower wheat powdery mildew control rates by DMI fungicides in recent seasons.
Wheat powdery mildew populations resistant to QoI fungicides are becoming widespread in the majority of wheat growing areas across Australian Eastern states. This resistance, which can be found at very high levels in some areas (Simpfendorfer et al., 2021) also affects other fungicides from the QoI group. Additionally, isolates carrying resistance to both QoI and DMI groups have been found at high frequencies in some locations.
Consequently, in regions where wheat powdery mildew resistance to DMI and QoI is in high frequencies, the practice of using SDHI fungicides for the control of other diseases could quickly lead to the emergence of resistance in this MoA group as well.
The Australian Fungicide Resistance Extension Network (AFREN), a GRDC investment, has identified five key actions aimed at avoiding and managing fungicide resistance in any crop and region:
- Avoid susceptible crop varieties
- Rotate crops – use time and distance to reduce disease carry-over
- Use non-chemical control methods to reduce disease pressure
- Spray only if necessary and apply strategically
- Rotate and mix fungicides/MoA groups
Unfortunately, the presence of DMI and QoI resistant wheat powdery mildew populations at high levels, together with the relatively lower activity of SDHIs against this disease and its higher associated risk for resistance development, makes the rotation and mixing of fungicides (action 5 above) from different MoA’s a very difficult task. In this scenario, actions 1-3 seem the logical approach, at least until disease levels drop and fungicides with higher activity against wheat powdery mildew become available to growers.
The present study provides an idea of the level of fungicide resistance exhibited by these DMI-resistant populations and to what extent the reliance on chemical management for the control of wheat powdery mildew is compromised.
Growers and agronomists concerned about DMI or QoI lack of performance against wheat powdery mildew should contact the CCDM’s Fungicide Resistance Group at frg@curtin.edu.au. Alternatively, contact a local regional plant pathologist or AFREN expert to discuss the situation. A list of contacts is on the AFREN website. Further information on fungicide resistance and its management in Australian grain crops is also available via the AFREN website.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through cooperation and the support of the GRDC, the author would like to thank them for their continued support.
References
ABARES (2020). Australian crop report. Australian Bureau of Agricultural and Resource Economics and Sciences. Canberra.
Kylie Ireland, Ciara Beard, John Cameron, Steven Chang, Jenny Davidson, Kejal Dodhia, Tara Garrard, Andrea Hills, Grant Holloway, Kith Jayasena, Levente Kiss, Wesley Mair, Steven Marcroft, Mack McLean, Andrew Milgate, Nick Poole, Steven Simpfendorfer, Lisle Snyman, Geoff Thomas, Hugh Wallwork, Angela Van de Wouw, Katherine Zulak, Francisco Lopez-Ruiz (2021). Fungicide resistance management in Australian grain crops. Grains Research and Development Corporation.
Kejal Dodhia, Belinda Cox, Richard Oliver and Fran Lopez-Ruiz (2021). Rapid in situ quantification of the strobilurin resistance mutation G143A in the wheat pathogen Blumeria graminis f. sp. tritici. Scientific Reports 11, 4526.
Gordon Murray and John Brennan (2009). Estimating disease losses to the Australian wheat industry. Australasian Plant Pathology 38, 558–570.
Sam Trengove, Stuart Sherriff, Jordan Bruce and Fran Lopez-Ruiz (2021). Management of powdery mildew on fungicide resistant wheat. GRDC Grains Research Update Adelaide.
Steven Simpfendorfer, Steven Chang and Fran Lopez-Ruiz (2021). Fungicide resistance in wheat powdery mildew across NSW and northern Victoria in 2020. GRDC Grains Research Update paper.
Contact details
Fran Lopez-Ruiz
Centre for Crop and Disease Management (CCDM), Curtin University
Kent Street, Building 304, Perth, Australia
Ph: 08 9266 3061
Email: fran.lopezruiz@curtin.edu.au
Date published: February 2023
® Registered trademark
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
GRDC Project Code: CUR1403-002BLX, DPI1706-010BLX, TRE2204-001RTX, DPI1607-009BLX, DPI2207-002RTX,