Managing sclerotinia stem rot of canola in 2023
Author: Kurt Lindbeck (NSW DPI), Ian Menz (NSW DPI), Steve Marcroft (Marcroft Grains Pathology) | Date: 14 Feb 2023
Take home messages
- Outbreaks of sclerotinia stem rot are sporadic and dependent on the growing season conditions. Saturated canopy conditions for more than 48 hours during flowering favour the development of the disease
- Outbreaks of sclerotinia stem rot were widespread in spring 2022 due to highly favourable conditions for the disease
- The frequency of canola or lupin in a paddock is very important in determining the risk of a sclerotinia outbreak, as these crops are very good hosts for the disease and can quickly build up levels of soil borne sclerotia
- Foliar fungicides for management of the disease are best applied at 20 – 30% bloom (15-20 flowers on the main stem) for main stem protection
- A bad Sclerotinia year will have a legacy effect for following broadleaf crops as the sclerotia can survive in soil for many years.
Where did Sclerotinia stem rot develop in 2022
The extraordinary rainfall conditions across southern NSW and Victoria in spring 2022 favoured the development of sclerotinia stem rot for an extended period. Ordinarily the conditions that favour development of the disease tend to cease in the second half of September as the air becomes warmer and drier. In 2022, wet conditions and saturated crop canopies continued well into November extending the disease pressure across many regions. The pre-emptive use of foliar fungicides by producers had the significant effect of reducing disease levels in commercial crops. The widespread wet conditions across the region resulted in sclerotinia stem rot developing in districts where the disease isn’t normally seen, and epidemics of the disease were able to develop from very low background pathogen levels.
How does the disease develop?
Sclerotinia stem rot is a complex disease with sporadic outbreaks due to the synchronisation and completion of various key development stages necessary for plant infection to occur. The pathogen responsible for this disease requires favourable weather conditions at every stage in its disease cycle. The stages of development include:
- Softening and germination of soil borne sclerotia
- Apothecia development and release of ascospores
- Infection of petals by air-borne ascospores
- Senescence of infected petals in the presence of moisture and subsequent stem infection.
Weather conditions during flowering play a major role in determining the development of the disease. The presence of moisture during flowering and petal fall will determine if sclerotinia stem rot develops. Dry conditions during this time can prevent development of the disease, hence even if flower petals are infected, dry conditions during petal fall will prevent stem infection development.
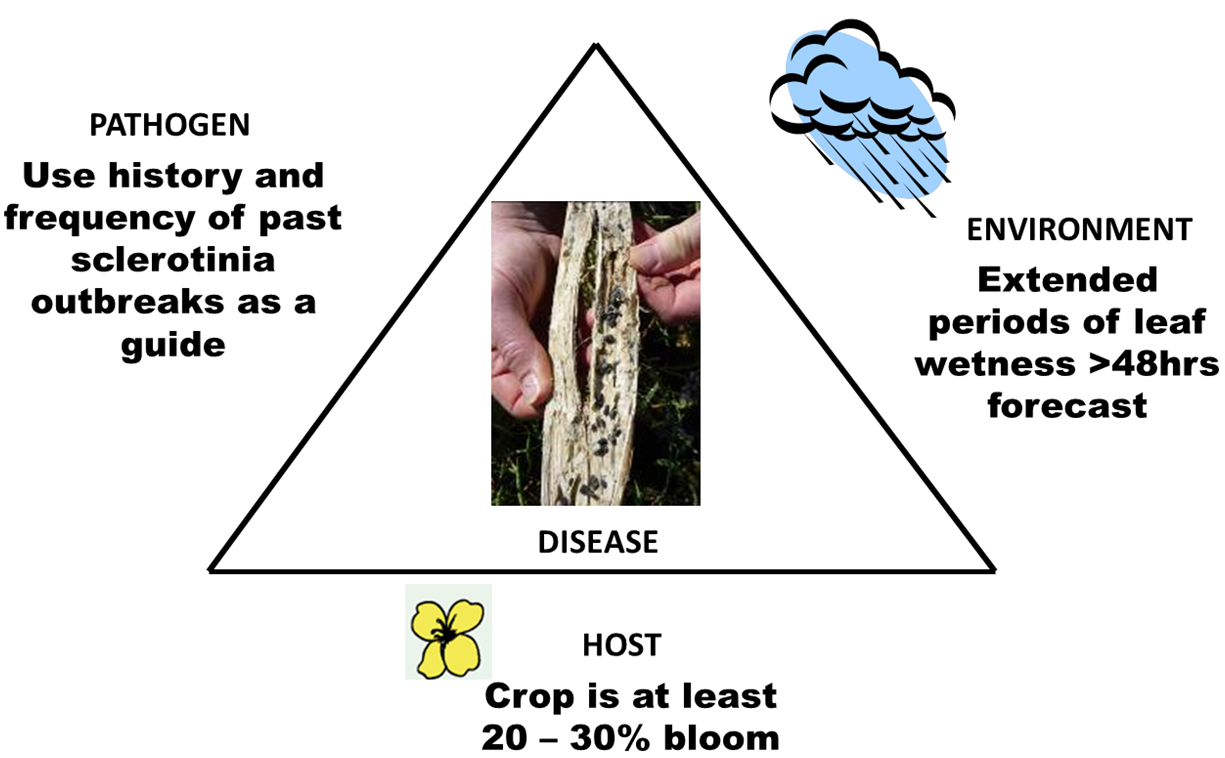
What are the factors that drive the development of sclerotinia stem rot?
- Frequency of sclerotinia outbreaks. The past frequency of sclerotinia stem rot outbreaks in the district can be used as a guide to the likelihood of sclerotinia developing this season. Paddocks with a recent history (last 5 years) of sclerotinia outbreaks are an indicator of potential risk, as well as those paddocks that are adjacent. The frequency of canola and lupin in the paddock can also increase disease risk. Canola and lupin are very effective hosts for the disease and can quickly build up levels of soil-borne sclerotia.
- Commencement of flowering. The timing of commencement of flowering can determine the severity of a sclerotinia outbreak. Spore release, petal infection and stem infection have a better chance of occurring when conditions are wet for extended periods, especially for more than 48 hours. Canola crops which flower earlier in winter (late June - July) are more prone to disease development and exposure to multiple infection events.
- Spring rainfall. Epidemics of sclerotinia stem rot occur in districts with reliable late winter and spring rainfall with long flowering periods for canola. These provide long periods of canopy wetness necessary for the disease to develop, at least 48 hours or more. Overnight dews generally won’t trigger epidemics of the disease.
Figure 1. Factors that drive the development of sclerotinia stem rot
Pre-sowing sclerotinia management
Crop rotation
- Grow canola only once in every 4 to 5 years to reduce build-up of sclerotia
- Incorporate lower-risk crops into the crop rotation e.g., cereals, field pea and faba bean
- Separate last year’s canola stubble and new seasons’ crops by at least 500m
- Ascospores of Sclerotinia spread within 100m to 400m of the apothecia.
Clean seed
- Always use seed free of sclerotia where possible
- Grade retained seed for sowing to remove sclerotia if in doubt
- Grain receival standards allow a maximum of 0.5 per cent sclerotes in the sample.
Variety selection
- There are no Australian canola varieties with known resistance to sclerotinia. Some differences may be observed in the level of stem rot in some seasons. This is likely to be related to the variety maturity, and timing of flowering with infection events.
Crop management
- Always follow the recommended sowing time and seeding rate for your region
- Early maturing varieties sown early can be prone to developing stem infection due to the earlier commencement of flowering when conditions are wet for prolonged periods
- Once flowering starts the crop becomes susceptible to infection and prolonged exposure to infested senescent petals means greater chance of stem infection
- Bulky crop canopies can retain more moisture and are conducive for the development of stem infections
- Wider row spacing or reduced seeding rates can increase air-flow through the canopy, reducing moisture retention and potential for infection.
Burning
- Burning stubble and windrows will kill some sclerotia, but will not significantly reduce the risk of disease
Use SclerotiniaCM app (see useful resources) to determine the most appropriate management strategies for your district.
Post-sowing sclerotinia management – fungicide application
- Use foliar fungicides to prevent early stem infection via infested petals
- Always use fungicide products that are currently registered in your state
- Timing of foliar fungicide application is more important than choice of fungicide product in reducing potential levels of stem infection
- Foliar fungicide application is most effective before an infection event
- Application of fungicide at 20-30% bloom is essential to reduce main stem infection and the majority of yield loss. Application at this stage protects early petals from infection and enables the fungicide to penetrate the canopy and protect potential infection sites where petals fall
- Multiple foliar fungicide applications may be needed in high disease-risk districts with high yield potential. Applications at both 10-20% and 50% bloom provide critical early and follow up protection from multiple infection events. Fungicide applications made during full bloom have limited penetration into the crop canopy and will not protect main stems from infection
- Use high water rates (at least 100 litres per hectare) to achieve adequate coverage and penetration into the crop canopy
- Foliar fungicides generally have an active life of two to three weeks. The protection provided may wear off during the critical infection period or where crops have an extended flowering period. A single fungicide application early may not be effective at preventing later infections
- Foliar fungicides have no effect on managing basal infections, as this occurs below the soil surface and beyond the activity of foliar fungicides. Foliar fungicides do not travel down the vascular tissue in plants.
ALWAYS:
- Determine disease risk as your crop enters the flowering period
- Assess bloom stage, seasonal conditions and weather forecasts to identify the potential risk periods to your crop
- Identify how many consecutive wet days are forecast as the crop commences flowering and the week ahead, especially consecutive wet days of 48 hours or more
- Monitor crops for disease development and identify the types of infection. Basal and main stem infections cause the most yield loss.
Useful resources
- NSW DPI winter crop variety sowing guide (disease updates, fungicide products)
- SclerotiniaCM App for iPad and android tablets
Acknowledgements
The authors wish to thank NSW DPI and GRDC for co-investment into this research.
Contact details
Kurt Lindbeck (Senior Pulse and Oilseed Pathologist)
NSW Department of Primary Industries, Wagga Wagga Agricultural Institute
Ph: 02 69 381 608
Email: kurt.lindbeck@dpi.nsw.gov.au
Steve Marcroft
Marcroft Grains Pathology
Grains Innovation Park
Natimuk Rd, Horsham VIC 3400
Mb: 0409 978 941
Email: Steve@grainspathology.com.au
GRDC Project Code: DPI2206-023RTX,
Was this page helpful?
YOUR FEEDBACK