Using zinc phosphide to control wild house mice
Using zinc phosphide to control wild house mice
Author: Steve Henry, Lyn A. Hinds, Wendy A. Ruscoe, Peter R. Brown, Nikki Van de Weyer, Freya Robinson (CSIRO Health and Biosecurity, Canberra), Richard P. Duncan (University of Canberra) | Date: 07 Feb 2023
Take home messages
- Mice are not as sensitive to zinc phosphide (ZnP) as was first reported in studies in the 1980s.
- 2mg of ZnP is required on each grain to deliver a lethal dose to a 15g mouse.
- Grain bait mixed at 50g ZnP/kg wheat is significantly more effective than bait mixed at the previously registered rate of 25g ZnP/kg wheat.
- Reducing background food could be critical to achieving effective bait uptake.
- Timely application of ZnP grain bait at the prescribed rate is vital for reducing the impact that mice have on crops at sowing.
- Strategic use of bait is more effective than frequent use of bait.
Background
The content of this paper relates primarily to the GRDC investment, Determining the effectiveness of zinc phosphide rodenticide bait in the presence of alternative food supply. Growers were reporting concerns regarding the effectiveness of commercially prepared zinc phosphide (ZnP) wheat-based baits. In response, we conducted three experiments to examine the efficacy of ZnP bait. The initial experiment set out to identify a more attractive bait substrate, but the results of this work identified unexpected questions regarding the sensitivity of mice to ZnP. The second experiment re-assessed the acute oral toxicity of ZnP for wild house mice. The results of this work showed a significant difference between the previously reported LD50 of 32.68mg ZnP/kg body weight and our re-calculated LD50 of 72–75mg ZnP/kg body weight. We then quantified the efficacy of the higher lethal dose (~2mg ZnP per grain) compared to the registered rate (~1mg ZnP per grain) in a field trial. The results suggest that a kill rate of >80% could be achieved 90% of the time for the higher rate compared to the registered rate for which an 80% kill rate would be observed only 20% of the time. These results are helping to inform how and when growers and agronomists manage mice in cropping systems in Australia.
Experiment 1: Effects of background food on alternative grain uptake and zinc phosphide efficacy in wild house mice.
The initial trial to determine what was driving the reduced efficacy of the bait sought to test potential new bait substrates that might be more attractive to mice.
Experiment 1a: Two choice grain preference
Mice were held on a background food type (barley, lentils or wheat) and then offered the choice of an alternative grain type (malt barley, durum wheat or lentils) for five nights. Mice displayed a strong preference towards cereal grains, with a slight preference towards malt barley.
Experiment 1b: Toxic bait take against different background grains
Mice were held on a background food type (lentils, barley or wheat) then offered ZnP-baited grain (25g ZnP/kg grain) for three consecutive nights. Mice consumed toxic bait grains regardless of bait substrate although background food type had a strong influence on the amount of toxic bait consumed. Most of the mice in this experiment consumed what was considered to be a lethal dose, however the mortality rate was significantly lower than expected (Table 1) (Henry et al. 2022). Furthermore, animals that consumed toxic grains and didn’t die, stopped eating toxic grains (that is, became averse).
Table 1: Percentage mortality from ZnP bait (25g ZnP/kg grain) and the average number of toxic grains consumed for each background food type on night one of the study (Henry et al. 2022).
Background food | n | Mortality (%) | Toxic grains eaten (av.) |
|---|---|---|---|
Lentils | 30 | 86 | 7.3 ± 2.5 |
Barley | 30 | 53 | 4.5 ± 2.9 |
Wheat | 30 | 47 | 2.1 ± 1.6 |
Bait substrate key results
Mortality was not as high as expected in mice that consumed toxic grains. The development of aversion was rapid although its duration is unknown. These results identified questions relating to the sensitivity of mice to ZnP (Henry et al. 2022). Had we been selecting for mice that were less sensitive to ZnP through frequent application of bait over a 20-year period? Or were mice just less sensitive to ZnP than had been reported in the past?
Experiment 2: Acute oral toxicity of zinc phosphide: an assessment for wild house mice (Mus musculus).
This experiment re-assessed the acute oral toxicity of ZnP for wild house mice using an oral gavage technique, where known doses of ZnP were delivered directly into the stomachs of mice. The responses of three different groups of mice were assessed and compared: (1) wild mice from an area where ZnP had been spread frequently (exposed), (2) wild mice from an area where ZnP had never been used (naïve), and (3) laboratory mice (Swiss outbred). The proportion of mice that died at each dose was used to calculate a dose-response curve for each of the groups of mice (Figure 1) (Hinds et al. 2022).
Acute oral toxicity key results
The results showed no significant differences in the sensitivity of any of the groups of mice to ZnP, indicating that there has been no selection for tolerant mice in areas where mice had frequent exposure to ZnP. However, there was a significant difference between the previously reported LD50 of 32.68mg ZnP/kg body weight (Li and Marsh 1988) and our re-calculated LD50 of 72–75mg ZnP/kg body weight. These results mean that 2mg of ZnP/grain is needed instead of 1mg of ZnP/grain to kill a 15g mouse (Hinds et al. 2022).
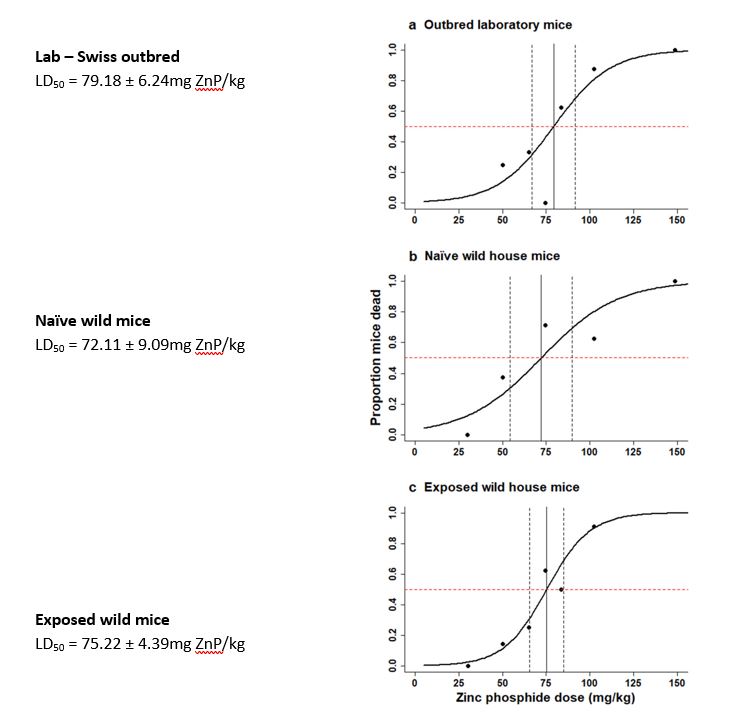
Figure 1. Proportion of mice dying after oral gavage with different ZnP concentrations (mg ZnP/kg body weight). Calculated dose response curves for (a) outbred laboratory mice, (b) naïve wild house mice, and (c) exposed wild house mice. Horizontal dashed line represents 50% mortality; vertical solid line equates to LD50 value; vertical dashed lines represent standard error for the LD50 estimate. N>four animals per test dose, with a mix of males and females (Hinds et al. 2022).
Experiment 3: Improved house mouse control in the field with a higher dose zinc phosphide bait.
This experiment addressed the efficacy of the two different bait types, ZnP25 (25g ZnP/kg bait, ~1mg ZnP/grain) applied at 1kg bait/ha and the new formulation, ZnP50 (50g ZnP/kg bait, ~2mg ZnP/grain), applied at 1kg bait/ha.
Nine sites were selected on farms in the area surrounding Parkes in central NSW, three un-baited control sites, three sites baited with ZnP25 (25g ZnP/kg bait), and three sites baited with ZnP50 (50g ZnP/kg bait). All sites were trapped prior to baiting to establish population sizes and then again after baiting to determine changes in population.
Field trial key results
Baiting with ZnP50 led to a median reduction in mouse numbers of >85%. Modelling showed that under similar circumstances, using the ZnP50 formulation should deliver >80% reduction in population size most (>90%) of the time. In contrast, the current registered bait (ZnP25) achieved approximately 70% reduction in population size, but with more variable results. We would be confident of getting an 80% reduction in population size only 20% of the time if using the currently registered ZnP25 bait under similar field conditions (Figure 2) (Ruscoe et al.2022).
Figure 2. The probability of achieving a certain reduction in population size or better by using the ZnP50 bait (solid black line) and the ZnP25 bait (solid grey line). The dotted vertical line shows that there is a ~90% chance of getting a >80% reduction in population size by using ZnP50, but only a 20% chance of achieving that outcome by using ZnP25 (Ruscoe et al.2022).
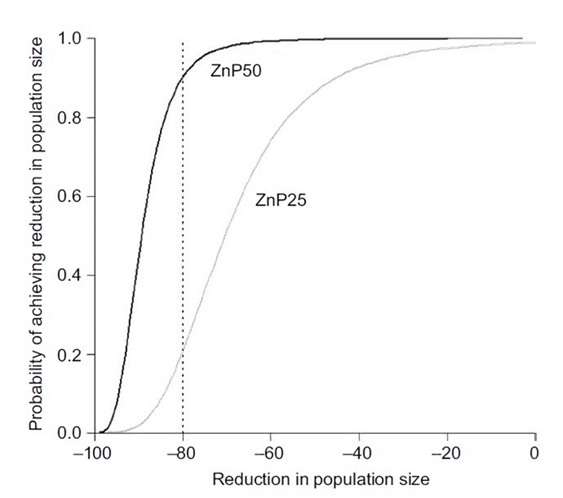
Figure 2. The probability of achieving a certain reduction in population size or better by using the ZnP50 bait (solid black line) and the ZnP25 bait (solid grey line). The dotted vertical line shows that there is a ~90% chance of getting a >80% reduction in population size by using ZnP50, but only a 20% chance of achieving that outcome by using ZnP25 (Ruscoe et al.2022).
Conclusion
- Mice are not as sensitive to ZnP as was first reported in studies in the 1980s.
- 2mg of ZnP is required on each grain to deliver a lethal dose to a 15g mouse.
- ZnP grain bait mixed at 50g ZnP/kg wheat is significantly more effective than bait mixed at the previously registered rate of 25g ZnP/kg wheat.
Future research
Substantial grain loss, pre- and post-harvest is common in zero and no-till cropping systems. In 2022, it was estimated that $300 million worth of grain (GRDC project code GGA2110-001SAX) was left on the ground post-harvest in WA alone and reports of losses of 1t/ha are not uncommon (pers. comm). Bait spread at 1kg/ha equates to approximately three toxic grains per square metre. If there have been losses of 1t/ha, equivalent to about 2200 grains per square metre, finding a toxic grain becomes a game of hide and seek for mice (Figure 3). Understanding the role that background food plays in the uptake of ZnP bait will be critical to achieving effective mouse control.
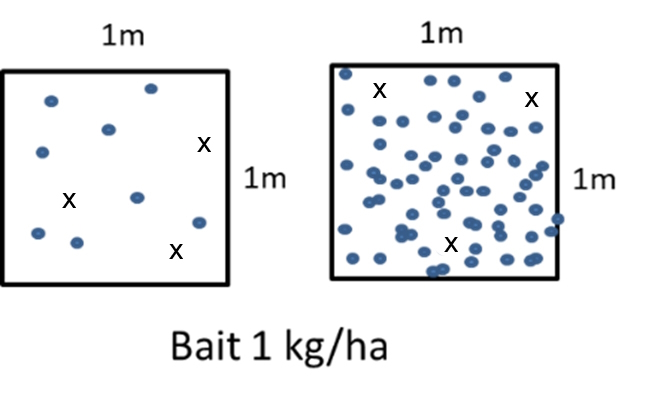
Figure 3. Representation of detectability of toxic grains at different levels of background food. The dots represent grains and crosses represent toxic grains.
Conclusion
- Mice are not as sensitive to ZnP as was first reported in studies in the 1980s.
- 2mg of ZnP is required on each grain to deliver a lethal dose to a 15g mouse.
- ZnP grain bait mixed at 50g ZnP/kg wheat is significantly more effective than bait mixed at the previously registered rate of 25g ZnP/kg wheat.
Future research
Substantial grain loss, pre- and post-harvest is common in zero and no-till cropping systems. In 2022, it was estimated that $300 million worth of grain (GRDC project code GGA2110-001SAX) was left on the ground post-harvest in WA alone and reports of losses of 1t/ha are not uncommon (pers. comm). Bait spread at 1kg/ha equates to approximately three toxic grains per square metre. If there have been losses of 1t/ha, equivalent to about 2200 grains per square metre, finding a toxic grain becomes a game of hide and seek for mice (Figure 3). Understanding the role that background food plays in the uptake of ZnP bait will be critical to achieving effective mouse control.
Figure 3. Representation of detectability of toxic grains at different levels of background food. The dots represent grains and crosses represent toxic grains.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support.
References
Henry S, Brown PR, Van de Weyer N, Robinson F, Hinds LA (2022) Effects of background food on alternative grain uptake and zinc phosphide efficacy in wild house mice. Pest Management Science 78, 1090–1098.
Hinds LA, Henry S, Van de Weyer N, Robinson F, Ruscoe WA, Brown PR (2022) Acute oral toxicity of zinc phosphide: an assessment for wild house mice (Mus musculus). Integrative Zoology 0, 1–13.
Li J, Marsh RE (1988) LD50 determination of zinc phosphide toxicity for house mice and albino laboratory mice. Proceedings of the Vertebrate Pest Conference (AC Crabb and RE Marsh (eds), Monterey, California, USA, March 1988, 13, 91-94.
Ruscoe WA, Brown PR, Hinds LA, Henry S, Van de Weyer N, Robinson F, Oh K, Duncan RP (2022) Improved house mouse control in the field with a higher dose zinc phosphide bait. Wildlife Research, doi 10.1071/WR22009.
White B, (2022) Measuring Harvester Losses in Western Australia. GRDC project code GGA2110-001SAX
Contact details
Steve Henry
@mousealert
CSIRO Health and Biosecurity
GPO Box 1700, Canberra ACT 2601
Ph: 02 6246 4088
Email: Steve.Henry@csiro.au
GRDC Project Code: CSP1804-012RTX,
