Fall armyworm: impact by crop, management strategy and resistance
Author: Melina Miles (DAF Qld), Adam Quade (DAF Qld), Joe Eyre (QAAFI-UQ), Lisa Bird (NSW DPI), Richard Sequeira (DAF Qld) | Date: 28 Feb 2023
Take home message
- Pre-flowering defoliation caused by fall armyworm (FAW) in maize for a prolonged period and during later vegetative crop stages, resulted in significant yield reduction
- In most maize and sorghum production areas, FAW activity is low in spring and increases as the summer progresses. Early planted crops have largely avoided significant FAW damage, and this has been an effective strategy for minimising spraying and potential yield loss
- Like Helicoverpa armigera, FAW has a strong capacity to develop insecticide resistance. An integrated management approach, and strategies to minimise selection pressure on FAW populations, will be critical for effective and economically sustainable management of this pest.
Introduction
Australian agricultural industries are now into the third summer season of monitoring and managing Fall armyworm (FAW) since its arrival in Australia in early 2020. Whilst just three seasons of data may seem inadequate for drawing conclusions compared with the 40+ years of R&D on Helicoverpa, for example - it has been imperative that impacted industries, growers and advisors are provided with practical information on how they can best monitor and manage FAW to minimise crop losses. This is not to say that the working rules of thumb being communicated to industry are without empirical basis, they have their foundations in ongoing research outcomes from local trials, consultation with overseas researchers, and Australian grower/agronomist experience.
What is presented in this paper is key information that will assist in understanding FAW behaviour in crops and the regions, and best-bet management strategies that will be refined as there is more research and experience with this new pest.
FAW activity
The most accessible data on FAW activity in different regions comes from the network of pheromone traps being operated by Queensland Department of Agriculture and Fisheries (QDAF) staff, agronomists and growers.
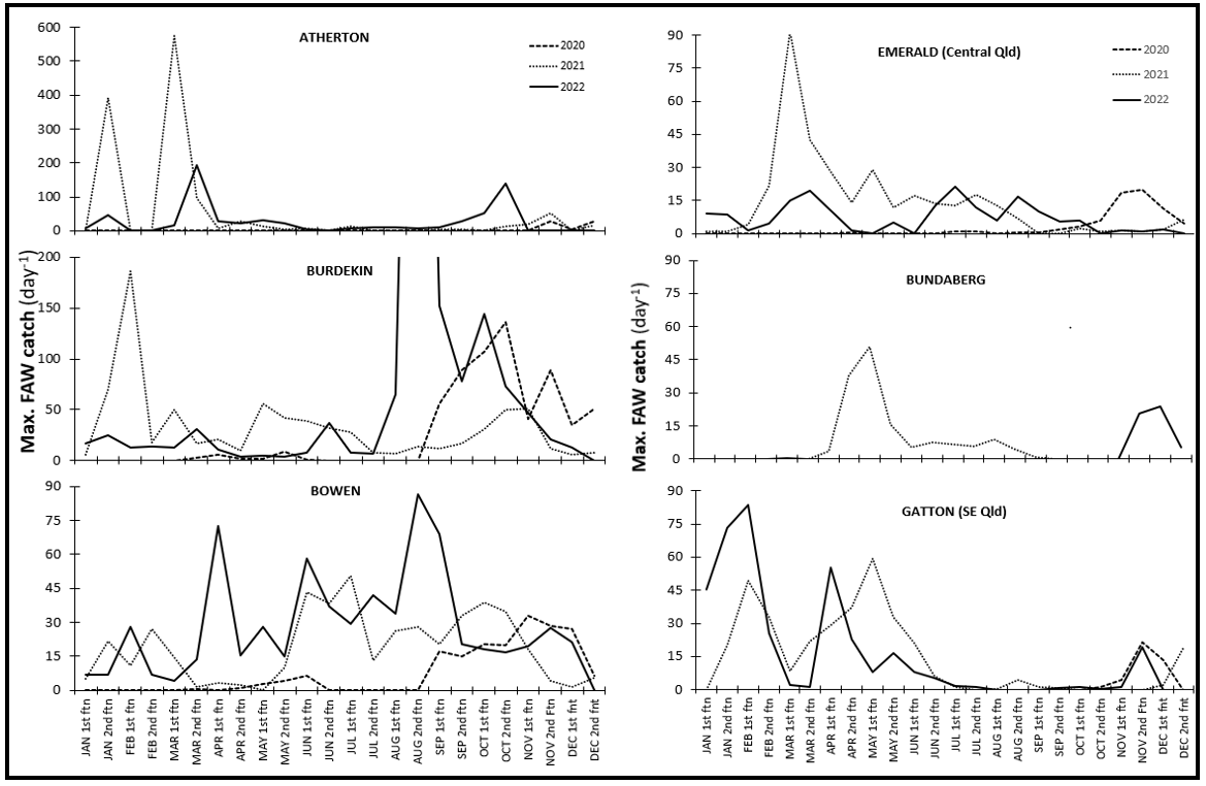
Important in interpreting the data presented in these graphs is that moth activity is a function of host availability (attractive crops in the ground where traps can be placed), night-time temperatures suitable for moth activity and moth abundance. In 2020-21 there were traps across Qld and NSW. In 2022-23, there is data from Qld only. A selection of trap data is presented here to illustrate key points.
Figure 1. Summarised pheromone trap data from representative locations from North Qld (Atherton, Burdekin, Bowen) to Central Qld (Emerald, Bundaberg) and southern Qld (Gatton) for three seasons 2020-2022. Trap catch data is presented as the maximum daily catch for each fortnight throughout the year. Note the different scale (y axis) for northern and central/southern regions.
Key points to note from the figure below (Figure 1) are:
- The level of activity in North Qld (Atherton, Burdekin, Bowen) is higher and more persistent throughout the year than it is in central and southern regions
- Periods of very low to no activity are recorded in all regions, driven by an absence of hosts and/or cooler conditions
- All regions experience a peak in moth activity/abundance in spring and summer
- Periods of rainfall suppress moth trap catches.
Susceptible crops that avoid the periods of highest FAW activity (late spring – summer) have experienced significantly lower levels of damage in most districts. For example, maize crops sown in Sept-Oct on the Darling Downs have largely escaped FAW infestations and required no insecticide treatment. In contrast, crops sown in Dec-Jan are subjected to damaging infestations from early vegetative stages, requiring spraying to ensure establishment and protect from yield loss. There is anecdotal evidence of local population build up to higher densities where there are successive crop plantings (spring and then summer) in a local area, or on individual farms.
Development of economic thresholds for FAW in maize and sorghum
GRDC has invested in research to investigate the relationship between FAW infestation and impacts on yield for sorghum and maize. This research is a collaboration between QDAF, UQ-QAAFI and Cesar Australia and is in its second year. The aim of this research is to identify the growth stages most susceptible to yield loss as a result of FAW infestation, in order to inform management decisions. Ultimately the project will make available to industry a tool that will allow growers and agronomists to forecast the potential loss based on agronomic factors and FAW impacts (APSIM simulation) for pre-anthesis (tasselling) crop stages. Additional studies on direct feeding damage to reproductive structures and grain will contribute to thresholds for the reproductive stages.
In field trials, maize and sorghum were sown at the Gatton and Ayr research stations in 2021-22 and naturally infested with FAW. When the crops had three fully expanded leaves (V3), FAW was controlled in plot at either the V6, V8, V10 or V12 growth stage, then all plots were maintained FAW free from V12 until harvest. Crop development, canopy light interception, FAW infestation and yield components were monitored throughout the growing season.
An example of the results being generated include the following findings for irrigated maize. Natural FAW infestations of a maize crop at Gatton in 2022 with 8 to 12 expanded leaves showed 25 to 30% yield losses. Yields were correlated with the extent of FAW defoliation quantified as the fraction of intercepted photosynthetically active radiation (fiPAR) at anthesis and the population density of fertile plants. The effects of FAW infestation during the canopy expansion and growth stage on yields could be simulated in APSIM by attenuating model co-efficient for the potential largest leaf size until fiPAR matched observed values.
This means that yield loss was either a result of direct FAW feeding on immature leaves, indirectly through limiting photosynthate supply to developing leaves, or both, resulting in reduced total canopy size. Reduced fertility due to direct effects of FAW feeding or shading of FAW stunted plants by neighbouring plants was a secondary contribution to yield loss. However, it is unclear if crop stage sensitivity is consistent across genotypes of different maturity groups, or crops growing at different rates relative to FAW.
There are clear differences in the overall impact of FAW defoliation on maize and sorghum, but both crops have shown reductions in yield trials.
Insecticide resistance in Australian FAW and strategies to reduce the risk to effective chemistry
In 2020-21, NSW DPI (Dr Lisa Bird) undertook a benchmarking insecticide toxicity study on FAW populations from across Australia and compared the performance of insecticides on FAW and Helicoverpa armigera. The results of this study showed that Australian FAW populations were moderately resistant to carbamates (Group 1A) and highly resistant to synthetic pyrethroids (Group 3A). There was no evidence of resistance to insecticides in Groups 28 (e.g. chlorantraniliprole, Vantacor®), 5 (spinosyns, e.g. SuccessNeo®) or 6 (emamectin benzoate, e.g. Affirm), but a natural tolerance to Group 22A (indoxacarb, e.g. Steward®). The full results of the baseline study are published (Bird et al., 2022).
The results of this baseline study have proven to be consistent with the field experience. Control failures have been reported where carbamates (e.g., methomyl) and synthetic pyrethroids have been used. Whilst there are current permits in place allowing use of these products, their use is not recommended. Application of the Groups 28, 5, 6 have been highly efficacious, and initial indications from the 2022-23 resistant monitoring (testing incomplete at Jan 2023) suggest no shift in susceptibility to these actives in Queensland populations tested.
Ongoing monitoring of Australian FAW populations will be critical in the early detection of resistance development, making it more likely that changes in insecticide use can be implemented in a timely manner to prevent rapid product failure. Whilst overall use of insecticides is low in the grains industry, the insecticides of importance in managing FAW are also registered for the control of Helicoverpa and several other insect pests in grain and horticultural crops, increasing the risk of resistance development through incidental exposure. The potential for FAW moths to move between regions, possibly taking resistance genes with them, heightens the importance of monitoring populations from across Australia.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
The Queensland Government has invested in a rapid response to support industries impacted by FAW (2020-23). This investment supports research, insecticide resistance monitoring (2022-23), extension and skills development for industry and researchers. The Cotton Research and Development Corporation (CRDC) provided financial support for the FAW resistance benchmarking study undertaken by NSW DPI.
Michael Ashcroft provides technical support to FAW field and laboratory activities, and Robyn Nobbs assists with FAW colony maintenance and pheromone trap monitoring. Richard Sequeira provided the synthesised pheromone trap catch data.
Resources/References
Bird L, Miles M, Quade A, Spafford H (2022). Insecticide resistance in Australian Spodoptera frugiperda (JE Smith) and development of testing procedures for resistance surveillance. PLoS ONE 17(2): e023677.
Contact details
Melina Miles
Queensland Department of Agriculture and Fisheries
203 Tor St, Toowoomba, Qld, 4350
Ph: 0407 113 306
Email: melina.miles@daf.qld.gov.au
Date published: March 2023
® Registered trademark
GRDC Project Code: DAQ2107-002RTX,
Was this page helpful?
YOUR FEEDBACK