Cereal disease update 2024
Author: Hari Dadu (Agriculture Victoria, Horsham), Grant Hollaway (Astute Ag, Horsham), Robert Park, Mumta Chhetri, (The University of Sydney), Wesley Mair, Fran Lopez Ruiz, Sheree Krige (CCDM, Curtin University) | Date: 20 Feb 2024
Take home messages
- Proactive disease management, which combines variety selection, paddock selection and appropriate fungicide use, provides proven sustainable and economic disease control.
- Septoria tritici blotch reduced grain yield in highly susceptible wheat varieties by 28% in the MRZ (Wimmera) and 13% in the LRZ (Mallee).
- Net form of net blotch (NFNB) is common in barley and caused grain yield loss of up to 18% in susceptible varieties.
- Fungicide resistance is now common in cereal pathogens in Southern Australia and so, strategies to manage cereal diseases under fungicide resistance environments are urgently required.
Background
Implementation of proactive strategies for the control of cereal diseases can prevent avoidable losses when seasonal conditions are suitable for disease. This paper provides an update on the latest research regarding cereal diseases for Victorian growers.
2023 in review
Disease pressure on cereal crops during 2023 was high. Substantial inoculum carry over from 2022 and wet conditions during winter prompted high disease severity in cereal crops and resulted in yield losses despite below average rainfall in spring. Septoria tritici blotch (STB), powdery mildew and stripe rust of wheat, net form, spot form of net blotch and leaf rust in barley were the most common cereal diseases in Victoria. Avoiding highly susceptible varieties reduced disease risk and yield losses. New detections of resistance to fungicides highlights the importance of adopting strategies to slow further resistance development.
Avoiding suckers for sustainable disease control
The most important component of an integrated disease management strategy by far is the avoidance of highly susceptible (or sucker) varieties. We often assume that, for genetic control, we need to grow resistant varieties (that is, those rated R, RMR or MR), when varieties with a rating of MS (and in some cases, MSS) or better will provide protection from loss, especially when the varieties are grown on a large scale.
As shown in Figure 1, the risk of yield loss increases exponentially with increasing susceptibility. This shows that avoiding highly susceptible varieties can result in large reductions in disease risk. Also, highly susceptible varieties produce large amounts of inoculum, which has implications not just for that season, but also for the next.
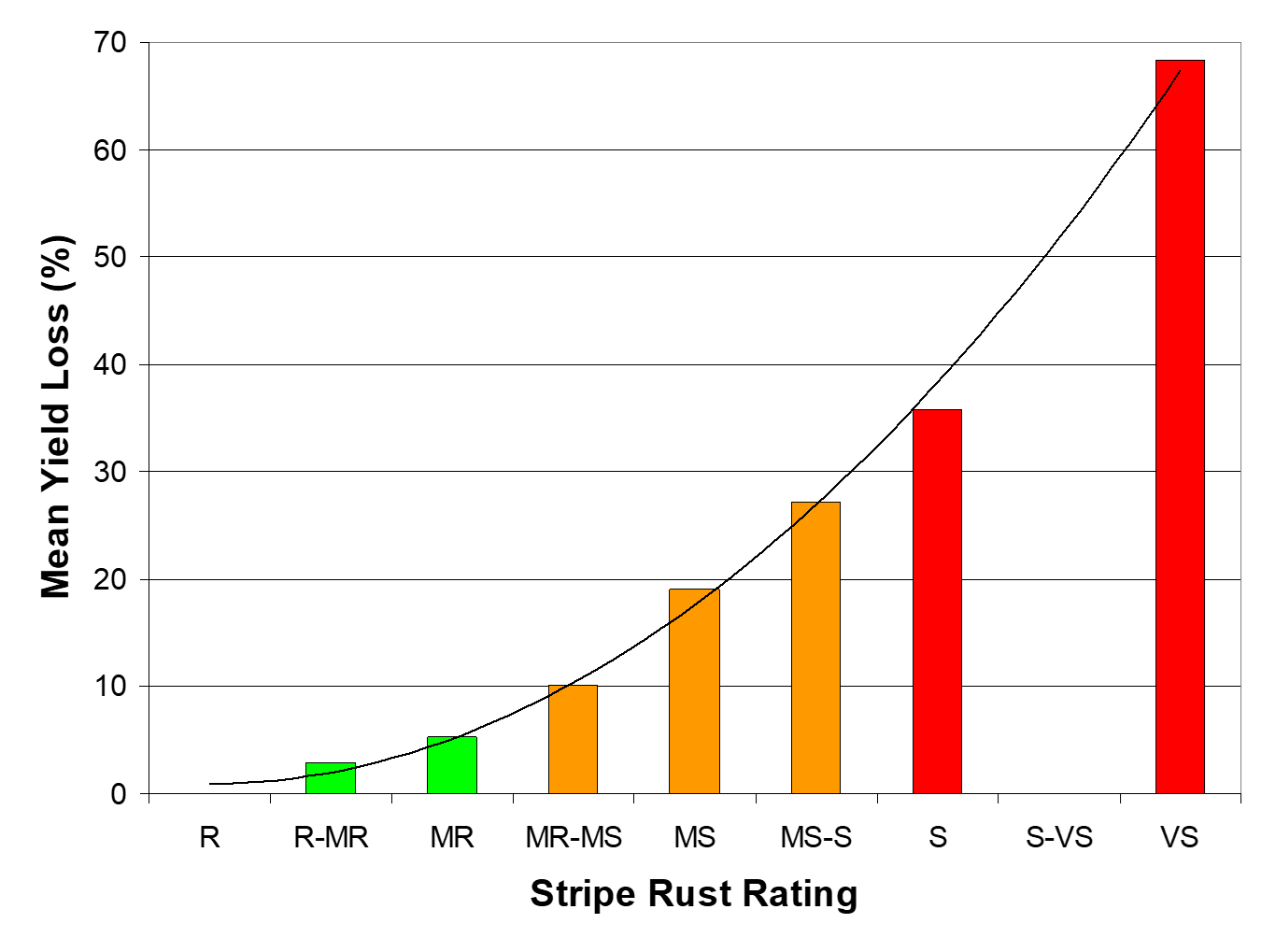
Figure 1. Varietal resistance rating and grain yield loss due to wheat stripe rust (mean of six sites across Vic, SA and NSW) in 2005.
Yellow leaf spot (YLS) in wheat is an example of how replacing highly susceptible varieties for example, Yitpi, LRPB Scout, LRPB Phantom) with partially susceptible varieties (for example, Scepter, RockStar, LRPB Trojan, Vixen) provided widespread disease control. Completely resistant varieties weren’t required.
Conversely, wheat powdery mildew is an example of where partially resistant varieties (for example, Yitpi, LRPB Scout, Axe) were replaced with highly susceptible varieties (for example, Scepter, RockStar, LRPB Trojan, Vixen, Corack, Wallup) on a large scale, resulting in powdery mildew becoming an important disease.
Rust update
Rust, in particular wheat stripe rust, was common in south-eastern Australia in 2023 due to the high levels of rust present in 2022 and its carry over on volunteer wheat growing over summer (the green-bridge). The common use of up-front treatment (for example, fungicide on fertiliser) provided good early suppression of disease, however high disease occurred when integrated control was not used.
With early summer rain events in many parts of the south-east, rust carry over on volunteer cereals is expected going into the 2024 season. Therefore, good rust management will be required with practices including:
- removing the green bridge (volunteer cereals) by mid-March
- using a current cereal disease guide to check resistance ratings of varieties and, where possible, avoiding susceptible varieties
- having a fungicide management plan, with an emphasis on up-front control options
- using the free StripeRustWM App; for iPads and tablets.
Internationally, Australia is in the enviable position of having excellent information on the national distribution of cereal rusts and their pathotypes (strains). This enables accurate disease resistance ratings for current and new varieties, and support for breeders in the development of resistant varieties. This surveillance by the University of Sydney, with GRDC’s support, during 2023 (until the end of November) received 289 samples of rust nationally, with results from 228 samples returned to date.
Wheat stripe rust
Pathotype analysis during 2023 identified four pathotypes of wheat stripe rust in eastern Australia: 239 E237 A- 17+ 33+ (90 isolates), 238 E191 A+ 17+33+ (47 isolates), 198 E16 A+ J+ T+ 17+ (19 isolates), and 238 E191 A- J+ T+ 17+ (12 isolates). Interestingly, the ‘238 pathotype’ (Pt. 238 E191 A+ 17+ 33+, first detected in 2021) was most common in northern Australia, while the ‘239 pathotype’ (2017) was most common in southern Australia. This difference in distribution may be due to the relative resistance/susceptibility of the varieties grown in the north and south to these two pathotypes. Due to the diversity in pathotypes present in eastern Australia, the resistance ratings in current disease guides reflect a ‘worse case’ against any of these four pathotypes.
Barley grass stipe rust
In 1998, a new specialised form (f. sp.) of stripe rust was detected on barley grass and a few barley varieties. This became known as ‘BGYR’ (‘Barley Grass Stripe (Yellow) Rust’) and does not infect wheat. In 2021, a new variant was detected (BGYR+) which has a large change in virulence that increased vulnerability in many varieties (for example, Capstan, Empress, Finniss, Keel, Ketch, Prior and Ulandra are all susceptible as seedlings). This new pathotype is now common in eastern Australia, with more reports of low levels of stripe rust in barley crops since 2021. Note that true barley stripe rust is still exotic to Australia.
The increasing occurrence of the BGYR+ pathotype on barley grass provides opportunity for it to undergo further changes in virulence. Ongoing monitoring and research to understand the vulnerability of barley varieties to potential future changes is vital in assessing and managing the risk it poses to the industry.
Barley leaf rust
During 2023, 33 samples of barley leaf rust were received from which three pathotypes were identified: 5457 P- (23 isolates), 5457 P+ (9 isolates), and 5656 P+ (4 isolates). All carry virulence for the resistance gene Rph3, which is in 20 barley varieties. Pathotypes 5457 P- and 5457 P+ belong to a single clonal lineage of the barley leaf rust population that was first detected in WA in 2001 and considered to be exotic. Since then, members of this lineage have dominated in Australia and now account for 89% of isolates. Work on fungicide insensitivity by the University of Sydney revealed that this lineage is insensitive to several DMI fungicides.
Septoria in wheat
Septoria tritici blotch (STB) has become the most widespread disease in wheat across Victoria, and yield and quality losses are now common in many parts of Victoria due to increased area of susceptible varieties and conditions conducive to disease. AgVic trials during 2023 demonstrated losses of up to 28% and 13% in susceptible varieties in the medium (Wimmera) and low rainfall (Mallee) regions, respectively (Table 1). Yield losses due to STB in the Mallee were reported for the first time. These trials clearly demonstrated the benefit of avoiding highly susceptible varieties in both the Wimmera and Mallee to reduce losses due to STB (Table 1).
Table 1: Septoria tritici blotch severity and grain yield of wheat varieties with (Max) and without (Min) disease at Longerenong (MRZ) and Kinnabulla (LRZ), Victoria, 2023.
Variety | Rating | Disease severityA (% leaf area affected) in Max. treatment | Grain yield (t/ha) | ||||||
|---|---|---|---|---|---|---|---|---|---|
Longerenong | Kinnabulla | Longerenong | Kinnabulla | ||||||
11 Sep Z59B | 8 Sep Z59 | Max.C | Min. | Loss (%)D | Max. | Min. | Loss (%) | ||
LRPB Lancer | MS | 10a | 5a | 5.87 | 6.16ns | 5 | 5.14 | 5.15ns | 0 |
Hammer CL Plus | MSS | 27b | 9b | 5.21 | 6.19** | 16 | 5.00 | 5.26** | 5 |
Scepter | S | 55d | 27de | 5.37 | 6.45** | 17 | 5.21 | 5.77** | 10 |
Calibre | S | 58d | 25cd | 5.74 | 6.60** | 13 | 4.76 | 5.44** | 13 |
Razor CL Plus | SVS | 70e | 29e | 3.87 | 5.35** | 28 | 4.01 | 4.38* | 8 |
LRPB Impala | SVS | 42c | 24c | 4.88 | 5.76** | 15 | 4.42 | 4.79* | 8 |
P | <0.001 | <0.001 | |||||||
Lsd (0.05) | 7.3 | 2.76 | |||||||
AWithin column means with one letter in common are not significantly different (0.05). ** = statistically significant at 5% and * = 10%. BDate of assessment made andZadoks growth stage. C Max. = Maximum disease treatment (No disease control with 1kg STB infected wheat stubble); Min. = Minimum disease treatment (No stubble, Seed (Fluquinconazole 167g/L @ 300mL/100kg seed) + Foliar applied fungicide at Z31 (Epoxiconazole 500g/L @ 125mL/ha) + Z39 (Benzovindiflupyr 40g/L + Propiconazole 250g/L @ 500mL/ha)). D Yield loss % for each variety was presented as % yield decrease vs the minimum disease treatment.
Where susceptible (S) or worse rated varieties are grown, fungicides may be required to protect yield. Consistent with previous research, two fungicide applications (at Z31 and Z39) increased grain yield by 16% in Wimmera and 10% in Mallee (Table 2). Always rotate fungicides with different modes of action to slow fungicide resistance development.
Table 2: Septoria tritici blotch severity (% leaf area affected) and yield loss in wheat (Scepter (S)) in response to fungicide treatments at Longerenong (MRZ) and at Kinnabulla (LRZ), Victoria, 2023.
Treatments | Longerenong | Kinnabulla | ||||
|---|---|---|---|---|---|---|
Disease severityA 11 Sep Z59 | Grain yield (t/ha) | Yield gain %B | Disease severity 8 Sep Z59 | Grain yield (t/ha) | Yield gain % | |
Untreated control | 58d | 4.78a | - | 31c | 4.98a | - |
Seed | 55d | 5.08ab | - | 31c | 5.02ab | - |
Foliar at Z31 | 33b | 5.16ab | - | 19b | 5.36cd | 7 |
Foliar at Z39 | 46c | 4.93a | - | 18b | 5.22bc | 5 |
Foliar at Z31 + Z39 | 30ab | 5.45b | 14 | 9a | 5.49d | 10 |
Seed + Foliar: Z31+Z39 | 26a | 5.55b | 16 | 9a | 5.49d | 10 |
P | <0.001 | 0.016 | <0.001 | <0.001 | ||
Lsd (0.05) | 7.2 | 0.47 | 2.15 | 0.22 | ||
AWithin a column, means with one letter in common are not significantly different at 0.05. BYield gain % is the percentage yield increase vs the untreated control. Fungicide treatments on seed (Fluquinconazole 167g/L @ 300mL/100kg seed) or foliar (Epoxiconazole 500g/L @ 125mL/ha at Z31 and Benzovindiflupyr + Propiconazole 500g/L @ 500mL/ha at Z39).
Net form of net blotch (NFNB) in barley
Net form of net blotch (NFNB) is a common foliar disease of barley in Victoria due to the adoption of susceptible varieties (for example, RGT Planet (SVS) and Spartacus CL (S)). During 2023, AgVic trials in the Wimmera (Wallup) demonstrated losses of up to 18% in the susceptible variety RGT Planet (SVS) (Table 3). The partial resistant variety Titan Ax (MS) had less infection and no yield loss, again showing the benefit of avoiding highly susceptible varieties.
Fungicides are an important part of NFNB control in susceptible varieties. Best economic control for NFNB management was provided by dual foliar application at Z31 and Z39 (Table 3). Earlier applications tend to provide most of the suppression in shorter season environments and later applications in longer high-rainfall environments. But unlike previous seasons, seed applied fluxapyroxad (an SDHI), the active ingredient in the seed treatment Systiva®, did not provide expected NFNB control due to resistance to this active. Likewise, field resistance and reduced sensitivity toward triazoles, such as tebuconazole and propiconazole, have also been increasing in frequency. See the section on fungicide resistance below for more details.
Table 3: Net form of net blotch severity (%) and yield gain in two barley varieties in response to different fungicide treatments at Wallup, Victoria, 2023.
Disease severityA (% leaf area affected) | RGT Planet (SVS) | Titan Ax (MS) | |||||
|---|---|---|---|---|---|---|---|
TreatmentB | RGT Planet (SVS) | Titan Ax (MS) | |||||
Z39 29/8 | Z82 10/10 | Z37 29/8 | Z82 10/10 | Grain Yield (t/ha) | Yield gain %B | Grain Yield (t/ha) | |
Untreated control | 10 | 41e | 3 | 1 | 4.41ab | - | 5.79 |
Seed | 9 | 34d | - | - | 4.52ab | - | - |
Foliar at Z31 | 2 | 23b | 0 | 0 | 5.10de | 18 | 5.99 |
Foliar at Z39 | 11 | 29cd | 3 | 1 | 4.70bc | - | 5.91 |
Foliar at Z55 | 11 | 29cd | - | - | 4.32a | - | - |
Foliar at Z31 + Z39 | 2 | 14a | 0 | 0 | 5.22e | 21 | 5.86 |
Seed + Foliar Z39 | 11 | 26bc | 0 | 0 | 4.87cd | 13 | 5.97 |
Seed + Foliar: Z31+Z39 | 2 | 12a | 0 | 0 | 5.29e | 22 | 6.24 |
P | <0.001 | <0.001 | <0.001 | 0.176 | <0.001 | 0.464 | |
Lsd (0.05) | 3.23 | 5.90 | 0.53 | ns | 0.34 | ns | |
AWithin a column, means with one letter in common are not significantly different at 0.05. BYield gain % based on percentage yield increase vs the minimum disease. Fungicide treatments on seed (Fluxapyroxad 333g/L @ 150mL/100kg seed) or foliar (Prothioconazole 210g/L +Tebuconazole 210g/L @300mL/ha)
Fungicide resistance
Resistance to fungicides is becoming an increasing threat to cereal crops across Australia. The status of resistance to fungicides in important cereal diseases is summarised in Table 4 and is based on work by the Fungicide Resistance Group (FRG) at the Centre for Crop and Disease Management (CCDM) and the University of Sydney’s rust program.
Table 4: Status of fungicide resistance and reduced sensitivity cases in Vic and SA cereal crops (Nov 2023).
StatusA | |||
|---|---|---|---|
Disease | Group 3 (DMI) | Group 7 (SDHI) | Group 11 (QoI) |
Barley | |||
Powdery mildew | Lab detection | Not detected | Not detected |
Net form net blotch | Reduced sensitivity | Field resistance | Reduced sensitivity (SA) |
Spot form net blotch | Reduced sensitivity | Not detected | Not detected |
Leaf rust | Reduced sensitivity | Not detected | Not detected |
Wheat | |||
Septoria tritici blotch | Reduced sensitivity | Not detected | Reduced sensitivity (SA) |
Powdery mildew | Field resistance | Field resistance | |
ALab detection - Measurable differences in sensitivity of the pathogen to the fungicide when tested in the laboratory. Detection of resistance in the lab can often be made before the fungicide’s performance is impacted in the field; Reduced sensitivity – Some reduction in fungicide performance which may not be noticed in the field. Serves as a warning that resistance is developing in the pathogen. Increased fungicide rates as per registered labels may be necessary. Field Resistance - Fungicide fails to provide an acceptable level of control of the target pathogen at full label rates.
Fungicide resistance management
There are five strategies that growers can adopt to slow the development of fungicide resistance and therefore, extend the longevity of the limited range of fungicides available:
- avoid susceptible crop varieties
- rotate crops
- use non-chemical control methods to reduce disease pressure
- spray only if necessary and apply strategically
- rotate and mix fungicides/modes of actions (MoA).
For more information on the management of fungicide resistance, consult the ‘Fungicide Resistance Management Guide’, available from www.afren.com.au. Following are the latest findings on fungicide resistance in Victoria:
Net form of net blotch in barley
During 2023, following reports of poor NFNB control with fungicides, particularly fluxapyroxad (a member of SDHI, Group 7), active ingredient in the seed treatment Systiva, and some compounds of DMIs (Group 3), samples (eight) from Victoria were sent to the CCDM for fungicide resistance testing. Six samples were positive for the C-S135R mutation (the second-highest levels of resistance to fluxapyroxad of the SDHI mutations found in Australian NFNB). Further testing showed that 41% and 22% of isolates grew on the highest dose of tebuconazole and fluxapyroxad, respectively (Table 5). This indicated the failure to provide disease control at the maximum label rate of these chemicals. Hence, strategies to manage NFNB under fungicide resistance environments are urgently needed.
Table 5: Percentage of isolates of net form of net blotch showing sensitivity/resistance to DMI (Group 3) and SDHI (Group 7) fungicides in Victoria during 2023.
Response | DMI (Group 3) | SDHI (Group 7) |
|---|---|---|
Sensitive | 54% | 41% |
Reduced sensitivity | 5% | 37% |
Resistant | 41% | 22% |
Septoria tritici blotch in wheat
Based on historic studies by NSW DPI, it is known that reduced sensitivity to the DMI (Group 3) fungicides is well established in Victoria. Work is required to establish the current status of resistance to these fungicides in Victoria.
The finding of resistance mutations to strobilurin fungicides (Qol, Group 11) in two Septoria tritici samples collected in South Australia during 2020 was very concerning. In vitro testing showed a 200-fold increase in resistance to azoxystrobin compared with the susceptible strain. Fortunately, testing of 25 samples collected from Victoria during 2023 did not detect this mutation, suggesting that it is still rare. Therefore, use of strategies to slow selection of this mutation are critical.
Conclusion
In the absence of proactive disease control, yield losses can be greater than 20%. It is, therefore, important that plans are developed to effectively manage cereal diseases this season. Disease management plans should consider paddock and variety selection and, where the risk warrants it, the proactive and prudent use of fungicides that avoid overuse to protect their longevity.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support. Funding for this work was provided by the Victorian Government (Agriculture Victoria) and the GRDC through the GRDC projects: DJPR2104-004RTX, DAQ2304-008RTX, DAW2112-002RTX and CUR2302-002RTX. Thanks to Agriculture Victoria’s Field Crops Pathology Team. Thanks also to the Birchip Cropping Group for field trials within the Victorian Wimmera and Mallee and to our research collaborators Drs McLean (Project Platypus / AgVic), Milgate (NSW DPI), Taylor (University of Adelaide) and Garrard (SARDI).
Useful resources
Septoria tritici blotch in wheat
Contact details
Grant Hollaway
Astute Ag
Horsham, VIC 3400
|0493 030 980
Grant.AstuteAg@gmail.com
@Grant_Hollaway
Hari Dadu
Agriculture Victoria
Private Bag 260, Horsham VIC 3401
03 5450 8301
Hari.Dadu@agriculture.vic.gov.au
@Imharidadu
GRDC Project Code: DJP2104-004RTX, DJP1905-002SAX, DJP2103-005RTX, DAQ2304-008RTX, DAW2112-002RTX, UOS2207-002RTX, CUR2302-002RTX,
Was this page helpful?
YOUR FEEDBACK
