Cereal rust update 2024
Cereal rust update 2024
Author: Robert Park (Sydney University), Mumta Chhetri (Sydney University), Yi Ding (Sydney University), Brad Baxter (NSW DPI), Hari Dadu (Agriculture Victoria) | Date: 14 Feb 2024
Take-home message
Vigilance and preparedness
- Timely detection remains crucial to combat rust threats from escalating.
- Destroying the green bridge is essential to prevent rust survival between cropping cycles.
- Proactive monitoring of vulnerable varieties and adjacent weedy grasses, and promptly respond by sending samples to the University of Sydney for pathotype analysis.
- Collaboration among researchers, farmers, breeders, and advisors is critical.
- Holistic strategies, encompassing resistant varieties and strategic fungicide application especially for fungicide insensitive pathogen isolates, are fundamental for comprehensive rust control and minimizing losses.
- Continuous backing for breeding programs to introduce diverse resistance traits in cereal varieties is paramount.
- Diversifying resistance genes is essential to counter evolving rust pathotypes effectively.
Collaboration for solutions
- Collaboration among researchers, farmers, breeders, and advisors is critical.
Integrated management is key
- Holistic strategies, encompassing resistant varieties and strategic fungicide application especially for fungicide insensitive pathogen isolates, are fundamental for comprehensive rust control and minimizing losses.
Resilience through diversification
- Continuous backing for breeding programs to introduce diverse resistance traits in cereal varieties is paramount.
- Diversifying resistance genes is essential to counter evolving rust pathotypes effectively.
Introduction
The University of Sydney's Plant Breeding Institute conducts a nationwide cereal rust survey and surveillance program, supported by GRDC (UOS2207-002RTX (9178966). This comprehensive initiative focuses on early detection of pathotypes by analysing cereal rust samples received from stakeholders, including farmers, advisors, and breeders. Working with state-based cereal pathologists, agronomists and other stakeholders, the Australian Cereal Rust Control Program monitors the occurrence and identity rust pathotypes in Australian cereal crops as an early warning scheme. The primary goal aims to enable risk management for the industry and guide breeding (Cereal Rust Report vol 20 Issue 3) and chemical decision interventions.
Wheat stripe rust
In 2023, wheat stripe rust (WYR) was detected as early as 7th July 2023, subsequent reports followed from Bethungra NSW (14th July), Tubbul NSW (20th July), Smeaton Victoria (20th July), Naracoorte SA (24th July), and Cressy/Longford Tasmania (26th July). Out of 309 cereal rust samples received, 215 samples were WYR. Four predominant pathotypes were detected this year, which were all detected in previous seasons: 198 E16 A+ J+ T+ 17+; 238 E191 A- J+ T+ 17+; 238 E191 A+ 17+33+; 239 E237 A- 17+ 33+. The dominance of the ‘239’ pathotype, particularly in southern regions (Victoria, South Australia, and Tasmania), has persisted throughout the year. Below is the summary of each pathotypes detected this year with distribution and frequencies from 2016 to 2023 detailed in Figures 1.
Pt. 198 E16 A- J+ T+ 17+ has decreased in frequency each year since 2020, only being detected in SA and NSW this year. It continues to impact vulnerable varieties such as Borlaug 100, DS Bennett, Illabo, LRPB Trojan, and Wedgetail.
Pt. 238 E191 A+ 17+ 33+, first detected in 2021, was again common in 2023, being isolated mostly from New South Wales and Queensland.
Pt. 238 E191 A- J+ T+ 17+, the Yr25-virulent derivative pathotype initially identified in 2022, was present at lower levels compared to the other three pathotypes in 2023. Nevertheless, its presence remains noteworthy for gaining insights into the dynamics of pathotype evolution.
In 2023, Pt. 239 E237 A- 17+ 33+ emerged as the dominant pathotype across eastern Australia, notably prevalent in the southern regions of Victoria, South Australia, and Tasmania. The observed dominance is likely associated with regional variations in wheat varieties, indicating that certain varieties are susceptible to specific pathotype groups. This pathotype poses a significant threat to wheat varieties, such as Catapult, Devil, Rockstar, Scepter, and Vixen. The frequent detection of this pathotype underscores the vulnerability of specific wheat varieties, emphasizing the critical importance of ongoing rust resistance breeding to effectively counter the threat posed by these pathotypes.
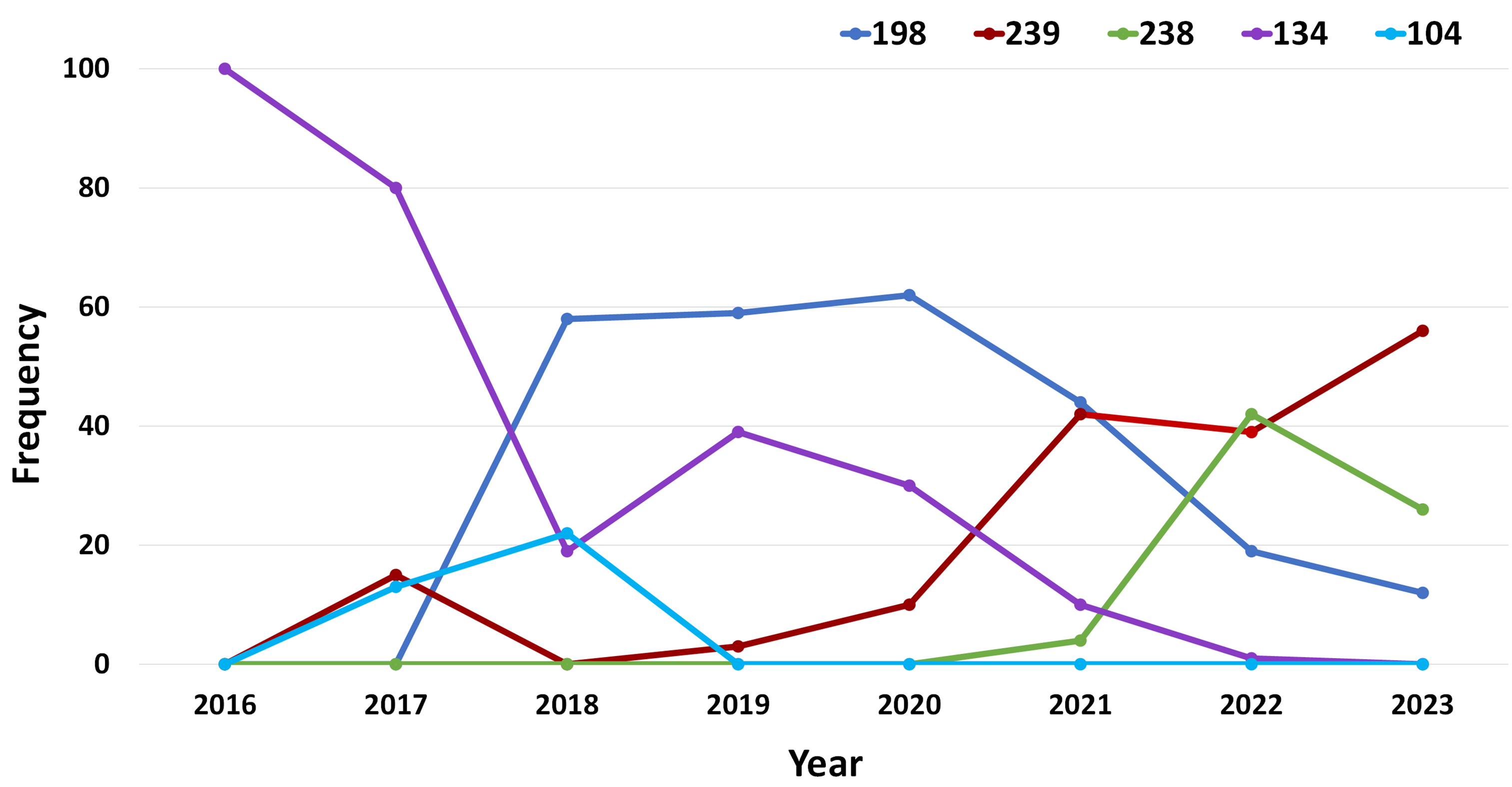
Figure 1. Frequency (%) of the five different pathotype groups of the wheat stripe rust pathogen in eastern Australia, 2016 through 2023
Barley grass stripe rust (BGYR)
Stripe rust, a fungal disease caused by puccinia striiformis, exhibits specialised variants for different crops. Puccinia striiformis f. sp. tritici (WYR) infects wheat and has been prevalent in Australian wheat crops since 1979. Another variant, puccinia striiformis f. sp. hordei (BYR), which is still not found in Australia, poses a significant threat to barley. A third form of p. striiformis, first detected in Australia in 1998, is colloquially known as BGYR (barley grass stripe [yellow] rust) and predominantly affects wild barley grass weed species such as hordeum glaucum and hordeum leporinum. Through whole genome sequencing, it has been determined that BGYR also exists in North America, infecting triticale and agropyron cristatum grass.
The emergence of the BGYR+ variant in 2021, showing increased virulence on barley, is a potential concern for the Australian barley industry. Greenhouse and field testing has raised significant concerns about the vulnerability of several current barley varieties, which should be monitored closely: Capstan, Charger, Empress, Explorer, Fandanga, Fathom, Finniss, Granger, Laperouse, Maritime, Moby, Neo, RGT Planet, Scope CL Plus, Shepherd and Spinnaker. BGYR+ has been notably severe on weedy barley grass, leading to substantial natural infections in nurseries at Horsham and Wagga in 2023, prompting worries about the potential spread from weedy barley grass.
In 2022, a significant shift in virulence for the BGYR+ pathotype occurred, with a variant assigned ‘BGYR+ A+’ gaining virulence for a resistance gene in the Avocet wheat variety. Additionally, recent fungicide insensitivity tests on 2022 surveyed samples found that the BGYR+ detected in 2021 and the mutant pathotype BGYR+ A+ in 2022 from New South Wales displayed insensitivity to fungicides. Despite the absence of registered fungicides specifically for BGYR control, it is concerning that none of the four demethylation inhibitor (DMI) fungicides tested (i.e. tebuconazole, prothioconazole, propiconazole, and triadimenol) were effective at the recommended high field rates of fungicides for other diseases against the two BGYR fungicide insensitive pathotypes (Figure 2.). While BGYR variants in barley crops have not caused yield losses, the consistent detection at low levels in certain barley crops over the past three seasons raises concerns on potential further changes in virulence. PBI is conducting further research to understand their effects and implications.

Figure 2. Tebuconazole applications at different rates vs BGYR Isolates (BGYR, BGYR+ and BGYR+A+,), where treatment 3 and Treatment 5 are recommended low and high field rates respectively.
In 2023, significant BGYR+ natural infections occurred in fields that will survive in green bridges, such as barley grass, wheat, and barley regrowth, allowing rust to survive between cropping cycles. This can lead to higher initial rust inoculum, causing rapid disease development. To minimise the risks, barley grass, wheat, and barley regrowth should be destroyed before cropping season begins.
Barley leaf rust (BLR) pathotypes and fungicide insensitivity
The 2023 survey received 33 samples of leaf-rusted barley, revealing three identified pathotypes: 5457 P- (23 isolates), 5457 P+ (9 isolates), and 5656 P+ (4 isolates). All these pathotypes exhibit virulence for the resistance gene Rph3, which was initially deployed in Australia in the Yarra cultivar and is currently present in 20 barley cultivars. The detection of virulence for Rph3 in 2009 has since become widespread in both eastern and western Australia. The 5457 P- and 5457 P+ pathotypes belong to a single clonal lineage of the Australian puccinia hordei population, initially detected in WA in 2001 and considered to have an exotic origin. This lineage has been dominant in Australian P. hordei populations, comprising 89% of all isolates pathotyped in 2023.
Research on fungicide insensitivity revealed that members of this lineage (5457 P- and 5457 P+) are insensitive to several DMI fungicides, raising concerns about the efficacy of these chemicals in controlling the identified rust pathotypes. The insensitive pathotype exhibited resistance to high field-rate concentrations of all seven DMIs (difenoconazole, epoxiconazole, propiconazole, tebuconazole, triadimenol, prothioconazole and Prosaro® (prothioconazole + tebuconazole)). Compound fungicides like Amistar® Xtra [DMI + QoI (quinone outside inhibitors); azoxystrobin + cyproconazole], Aviator® Xpro® a mixture of DMI + SDHI (succinate dehydrogenase inhibitors; prothioconazole + bixafen) and Radial® (DMI + QoI; azoxystrobin + epoxiconazole) effectively control the insensitive pathotype at high field rates with combined modes of action. Fungicide insensitivity is linked to copy number variation at the PhCYP51 locus, making it necessary to be cautious when using these chemicals due to their dynamic nature and adaptability to mixed modes of action.
Wheat leaf rust (WLR) pathotype and fungicide insensitivity
Out of 15 wheat leaf rust samples, 14 were identified as pt. 104-1,3,4,5,7,9,10,12 +Lr37 pathotype, while one sample, from South Australia, was classified as pt. 76-1,3,5,7,9,10,12,13 +Lr37.
Lr27+31, a complementary ASR leaf rust resistance gene, has been used in Australian wheat breeding since Gatcher was released in 1969. The emergence of virulence for ASR leaf rust resistance genes Lr13 and Lr27+31 has led to increased susceptibility to leaf rust in many varieties, including Corack, Emu Rock, and Wyalkatchem. Pathotype 104-1,3,4,6,7,8,10,12 +Lr37 combines virulence for these genes, making varieties carrying one or more of these resistances more susceptible in eastern Australia and Western Australia.
The fungicide insensitivity research on the WLR pathotypes revealed that 93-3,4,7.10,12, identified in 2020, exhibited insensitivity to recommended high doses of nine fungicides. However, compound fungicides like Amistar Xtra and Radial both with DMI + QoI modes of action effectively control this insensitive pathotype at high field rates. However, caution is advised when deploying two fungicides extensively, as this can encourage mutations causing fungicide insensitivity for compound chemicals.
A brief overview: understanding fungicide resistance in cereal rust pathogens.
1. Barley leaf rust (BLR) fungicide-insensitive pathotypes
- The BLR fungicide-insensitive pathotypes trace their origins back to the 5453 P clonal lineage identified in Western Australia in 2001
- Prevalent across all Australian barley-growing regions, exhibiting increased insensitivity.
- Display resistance to all eight DMIs at high field-rate concentrations
- Compound fungicides like Amistar Xtra (DMI + QoI), Aviator Xpro (DMI + SDHI), and Radial (DMI + QoI) effectively control insensitivity at high field rates with combined modes of action.
- Caution advised in extensive use of compound fungicides due to the potential for fungal adaptation to mixed modes of action.
2. Wheat leaf rust pathotype 93-3,4,7,10,12
- The wheat leaf rust fungicide-insensitive pathotypes 93-3,4,7,10,12 was identified in 2020
- Shows insensitivity to recommended high rates of nine fungicides.
- Amistar Xtra (DMI + QoI) and Radial (DMI + QoI) effectively control this pathotype at high field rates but warrant careful application to avoid further resistance.
3. Barley grass stripe rust (BGYR) pathotype
- Displays insensitivity to all tested DMI fungicides (tebuconazole, prothioconazole, propiconazole, triadimenol) at recommended high field doses.
- Infects not only barley grasses but also specific wheat and barley lines, making management complex.
Recommendations for industry safeguarding fungicide insensitivity
- Immediate and continuous research on fungicide insensitivity is critical as it's anticipated to pose a significant challenge in the future.
- Collaborative efforts among researchers, farmers, advisors, breeders, and donors are crucial to manage and mitigate the impact of insensitive pathotypes.
- Grain growers are urged to judiciously use fungicides considering the dynamic nature of fungal pathogens and their ability to adapt to various fungicidal modes of action.
Useful resources
Australian cereal rust survey and reports
Acknowledgements
The rust surveillance program for cereals across the nation, supported by GRDC (UOS2207-002RTX) at the University of Sydney's Plant Breeding Institute, extends gratitude to all collaborators including grain growers and agronomists who submitted rust samples from wheat, barley, oat, triticale, and cereal rye. Special recognition goes out to the ongoing support from Drs Lisle Snyman, Andrew Milgate, Brad Baxter, Steven Simpfendorfer, Grant Hollaway, Hari Dadu, Tara Garrard, Manisha Shankar, and Geoff Thomas. Additionally, we want to extend our appreciation to Wes Amor and Tom McGuire at Bayer CropScience Pty Ltd for supplying the fungicides for testing.
**Kindly send freshly collected rust samples solely in paper envelopes to the Australian Cereal Rust Survey, University of Sydney, Reply Paid 88076, Narellan, NSW, 2567.
Contact details
Robert Park
The University of Sydney, Faculty of Science
School of Life and Environmental Sciences
Plant Breeding Institute, Camden, NSW 2006
Ph: 0414 430 341
Email: Robert.park@sydney.edu.au
Twitter: @PbiCobbitty
Mumta Chhetri
The University of Sydney, Faculty of Science
School of Life and Environmental Sciences
Plant Breeding Institute, Camden, NSW 2006
Ph: 0404 392959
Email: Mumta.chhetri@sydney.edu.au
Twitter: @PbiCobbitty
Date published
February 2024
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
® Registered Trademark
GRDC Project Code: UOS2207-002RTX,
