Efficiently limiting yield loss from net-blotch in barley – a meta-analysis
Efficiently limiting yield loss from net-blotch in barley – a meta-analysis
Author: Paul Melloy (UQ), Kith Jayasena (DPIRD), Mark McLean (DPIRD), Lisle Snyman (DAF Qld), Geoff Thomas (DPIRD), Andrea Hills (DPIRD), Victor Galea (UQ), Jean Galloway (DPIRD) | Date: 29 Feb 2024
Take home message
- In marginal barley cropping regions where yield targets are below or equal to 3.4 tons per hectare, using foliar fungicides to control net-blotch might not be economically beneficial
- The most effective period for applying foliar fungicide to minimise yield loss is during and immediately after the emergence of the top three leaves (flag leaf, flag leaf -1 and flag leaf -2)
- Two fungicide applications timed between Z35 and Z60 provided the best yield protection
- Efficacy of spray programs to protect yield don’t tend to differ between the two net-blotch forms, SFNB (Spot Form Net Blotch) and NFNB (Net Form Net Blotch)
- Consult the ‘NetBlotchBM’ app when considering a disease management program.
Decision support tools
Effective integrated disease management in agriculture can lead to higher economic gains for growers. However, it demands a thorough understanding of the interactions between host plants and pathogens, and the time to gather data on the crop and make decisions to the economic thresholds for intervention. Decision support tools package the knowledge and economic thresholds for intervention to streamline integrated crop management programs. Decision support tools released by the Department of Primary Industries and Regional Development (DPIRD) in Western Australia are the result of collaborative efforts with disease experts from various Australian grain growing regions. They are founded on extensive field trial data, accumulated over decades. This presentation outlines some of the development process of the recently released Barley Net Blotch Manager (NetBlotchBM) by featuring insights from a meta-analysis of 111 field trials conducted over the past 20 years.
Net-blotch biology and background
Net blotch is a stubble-borne disease which constrains barley yields worldwide, potentially causing up to 44% in yield loss (Jayasena et al., 2007). The causal agent is characterised by two forms: net-form (NFNB) Pyrenophora teres f. teres, and spot form (SFNB) P. teres f. maculata (Ptm). The two strains differ in their symptoms, distribution, optimum environmental conditions, and likelihood of seed transmission (McLean et al., 2009). Despite some differences between the two ‘forms’, many similarities in the epidemiology remain the same.
Primary net-blotch infections in barley predominantly arise due to ascospore dispersals originating from infected stubble from previous seasons. However, primary infection can also occur from seed transmission (NFNB) and spore dispersals from alternative hosts (McLean et al., 2009; Joergensen 1980).
Ascospore production from stubble is reliant on the presence of moisture with an optimal temperature range between 15 and 20°C. Infection can occur rapidly, especially when leaves remain moist for extended periods. Visible symptoms typically emerge within 48 hours post infection and become more pronounced between 7 to 10 days post infection. This stage precedes the generation of secondary inoculum via conidia, which occurs approximately 14 to 20 days after the initial infection.
Disease progression occurs within a wide temperature range (8–33°C) with its epidemiology impacted by average temperatures. As the season advances, warmer temperatures tend to increase the levels of airborne conidia, potentially leading to more frequent infection cycles (Van den Berg and Rossnagel, 1991). However, without frequent high humidity or periods of sufficient leaf wetness, epidemics can stall, giving time for the host to grow through infections (Deadman and Cooke 1987).
Wet conditions significantly accelerate the buildup of net-blotch in barley, leading to greater disease-related losses in medium to high rainfall areas (McLean et al., 2022). Consequently, grain yield losses in these zones typically range between 4–25%, on average. In contrast, areas and seasons with lower rainfall tend to experience less impact, reporting average yield losses of 1–5% due to the disease (Khan 1989; McLean et al., 2016).
Net blotch primarily affects grain quality before detectable losses in grain quantity (McLean et al., 2016) complicating the assessment of economic losses. Yield loss predictions from disease severity depend on the timing of assessment relative to the crop's growth stage. Jayasena et al. (2002) cites the milky ripe stage (Zadoks Z73) as the optimum single crop growth stage where disease severity corelates with yield loss. The timing of initial infection also plays a role, presenting challenges in timing fungicide applications effectively. Mitigating yield loss to net blotch requires protecting the upper canopy leaves (flag leaf, flag -1 and flag -2) which contribute up to 72% of their photosynthates to grain production (McLean et al., 2009). To-date, application of foliar fungicides are therefore recommended between stem elongation (Z31) and flag leaf emergence (Z39) (McLean and Hollaway 2019; McLean et al., 2022).
Barley seasons with sub-optimal rainfall, or conditions not conducive to achieving maximum yield potential, are less likely to benefit financially from fungicide applications for net-blotch control. Additionally, these sub-optimal conditions often do not favour severe net-blotch infections. Barley yield thresholds, where intervention with a fungicide application is economically justified by loss in potential yield, remain uncertain and require further research.
Spatial factors, such as agro-ecological cropping zones in Australia, which characterise typical barley yield potential and common climate trends may provide additional insight into net-blotch management strategies. In this study we aim to identify yield thresholds where fungicide application results in significant yield protection for different agro-ecological zones corresponding with barley production areas in Australia.
Methods
We undertook a systematic review approach to this meta-analysis when incorporating data provided by collaborating institutions, DPIRD, Agriculture Victoria and Queensland Department of Agriculture and Fisheries. Suitability of the field trial data required it to meet the following criteria: sourced from a barley field trial on net-blotch undertaken in an Australian agro-ecological zone, a stubble inoculated trial, barley yield and variance between treatment replicates, and details of any disease management treatments, such as seed treatments, foliar fungicides, timing of foliar fungicide application and fungicide active ingredients. Trials not meeting the inclusion criteria were dropped before the analysis. Data from 111 trials were obtained and 29 were not included in the analyses for not meeting the inclusion criteria.
From the included trials, disease severity was measured as the percent leaf area diseased, however between trials disease severity was assessed at varying crop growth stages. To standardise the disease severity observations across all trials, we used a critical point approach where severity was estimated at ear emergence (Z50) in the no spray control plot. Area under the disease progress could not be used because the first and last severity ratings were rarely observed at the same growth stages. Because the aim was to determine the effect of the trial disease pressure on yield loss, and not correlating disease severity with individual treatments, the unsprayed control was used in categorising disease pressure for the trial.
The meta-analysis on actual yield evaluated treatment effects of the following fixed effect variables: categories representing foliar fungicide timing and frequency; disease pressure (percent leaf area diseased in the unsprayed controls); seed treatments; and the presence of inoculum on net-blotch infested stubble. Additional ‘random effects’ including barley genotype, net-blotch form, and nested spatio-temporal structured variables agro-ecological zone, trial location and trial id were evaluated for their effect on yield.
Table 1. Simplified discrete foliar fungicide treatments used in the meta-analysis.
Foliar fungicide* treatment timings | Zadok’s stages |
|---|---|
Early | <Z30 |
Node | Z30 – Z32 |
Stem extension | Z33 – Z35 |
Flag leaf | Z36 – Z39 |
Boot | Z40 – Z49 |
Head emergence | Z50 – Z59 |
Post-anthesis | >Z60 |
*Only group 3 – demethylase inhibitors were incorporated in the meta-analysis
Results and discussion
Preface
It is important to note the complexity of the meta-analysis undertaken here. The collated data testing timing of different fungicide treatments were derived from numerous independent trial studies, each subjected to differences in agro-ecological zones, trial location, disease pressure, seasonal rainfall, barley variety, inherent yield potential for the crop in that season, and sampling sizes. The number of trials testing the different timing of fungicide treatments in this meta-analyses and whether data are included from a balanced representation of these variables or primarily from a skewed number of variable categories leading to bias, will be discussed on presentation. However, overall, our meta-analysis does reveal trends allowing us to inform growers on likely best practice in managing net blotch in barley crops, while also highlighting key areas where more data generation would help improve the Barley Net Blotch Manager.
Yield summary
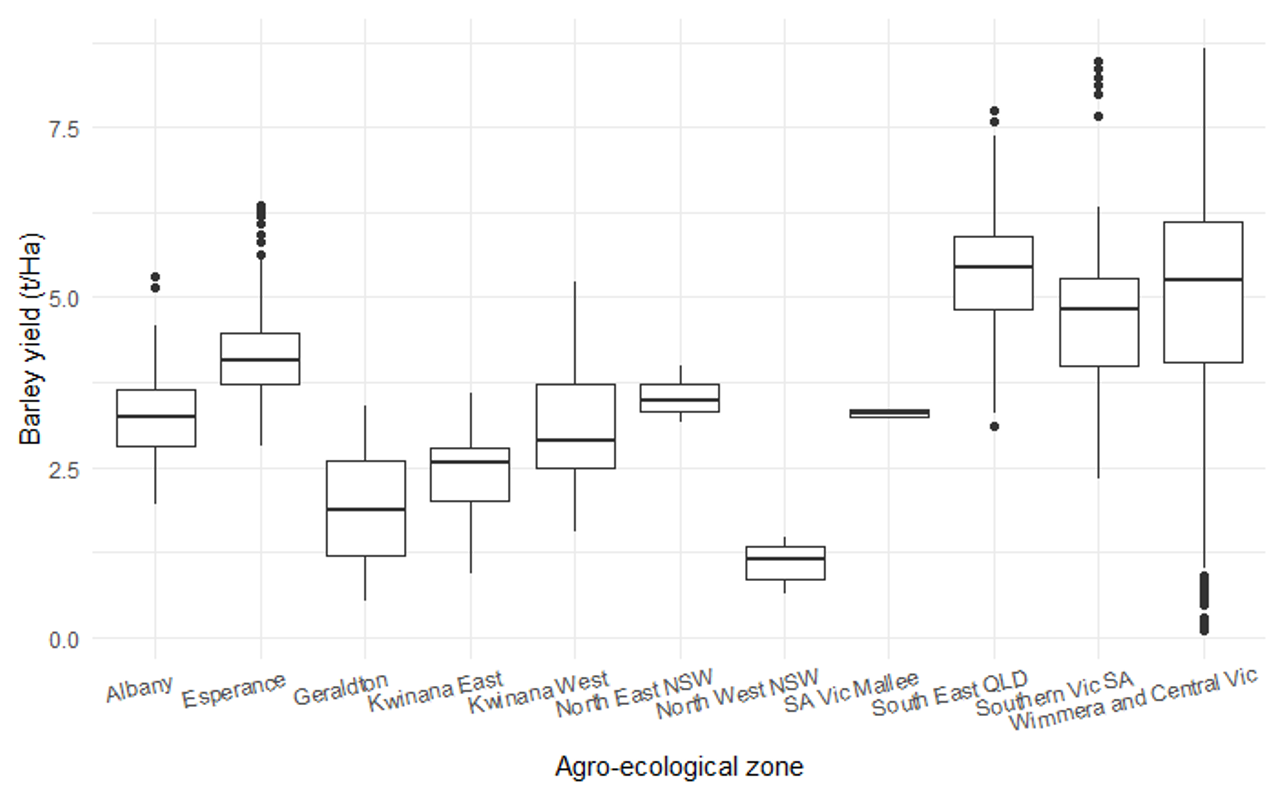
Raw yields varied between 0.09 t/ha to 8.67 t/ha, with just over half the trials yielding greater than 4 t/ha. Raw data indicated yields were likely to vary according to agro-ecological region (Figure 1).
Fungicide timing
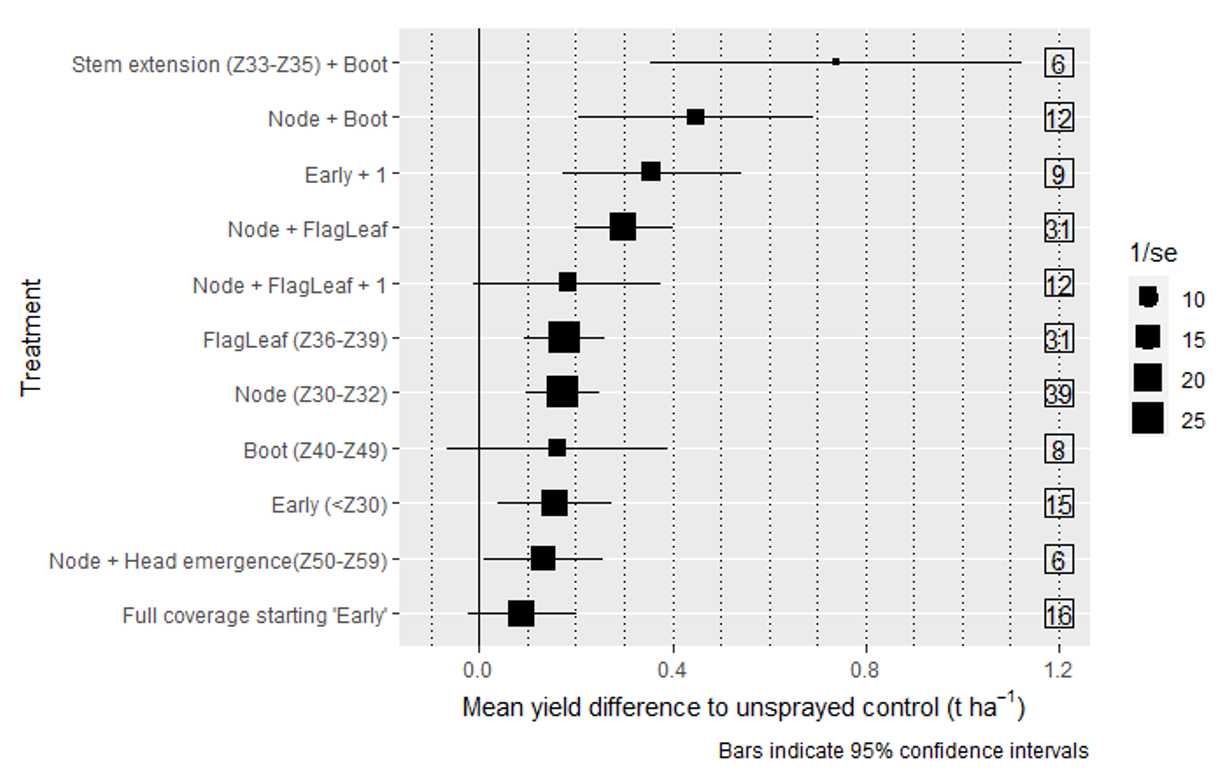
Two foliar fungicide applications with one spray at stem extension (Z33–Z35) and one at booting (Z40–Z49) was estimated to protect the most yield (0.692 t/ha; trials = 6). However this result should be viewed with scrutiny due to only six trials featuring this treatment. The next best were two-application regimes at: node development (Z30–Z33) plus booting (Z40-Z49) (0.460 t/ha; trials = 12), and the other occurring at ‘early’ (<Z29) plus one at head emergence (<Z50) (0.370 t/ha; trials = 9) (Figure 2). A two-spray regime starting with an application at node development and a second at flag-leaf emergence (Z37–Z39) protected an estimated 0.293 t/ha (trials = 31), with no additional benefit on yield compared to when a third spray was applied between boot (Z40–Z49) and post-anthesis (>Z60) (0.222 t/ha; trials = 12).
While there was no significant difference between any of the single spray scenarios, Node (Z30–Z32) on average protected the most yield (0.1737 t/ha; trials = 39). Followed by flag-leaf (0.17 t/ha, trials = 31), boot, (0.16 t/ha, trials = 8 and early (0.12 t/ha, trials = 15). Unexpectantly, treatments with full coverage starting ‘early’ showed no yield increase compared to unsprayed controls (0.089 t/ha, trials = 16). On closer inspection of the raw data, we found 11 of the 16 trials which included full coverage starting ‘early’ yielded less than 3 t/ha. Four of the five remaining trials which yielded greater showed no significant yield differences between treatments, while also recording low disease pressure. More higher yielding studies with heavy disease pressure are needed to understand the true effect of this treatment.
Figure 1. Boxplot of average barley yield in experiments for each agro-ecological zone. Boxes indicate the 25 and 75% quantiles, the central bar indicates the median, vertical lines indicate the 99% range of yields, dots show potential outliers.
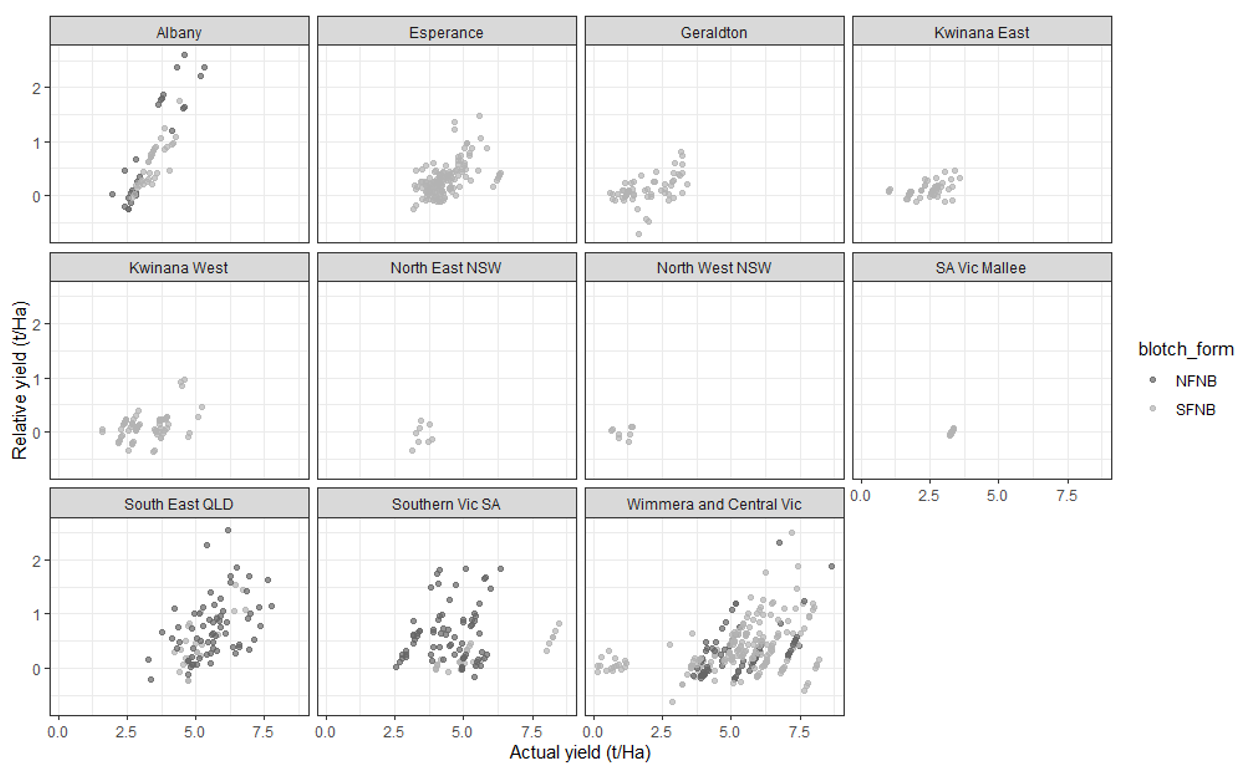
While differences between net-blotch form (net-form vs spot-form) were present in individual trials in different agro-ecological zones (Figure 3), the analysis showed no difference on their impact on crop yield (P> = 0.7273). Agro-ecological zone accounted for a large and significant amount of the barley yield variation, followed by rainfall and genotype. Locations within agro-ecological zone and variation between or within trials produced lower, albeit important, effects on yield. The efficacy of the foliar fungicide category was dependant on the individual field trial (P <0.001) when accounting for agro-ecological zone and location, and therefore likely indicates the differences between growing seasons.
Figure 2. Meta-analysis estimates of mean difference compared to unsprayed (no fungicide) control. Bars indicate 95% confidence intervals. Numbers in the boxes indicate the number of trials of each respective treatment included in the meta-analysis, 1/se is the inverse of the standard error graphed as the size of the point, the smaller the point the larger the uncertainty in the estimation. Treatment labels with ‘+1’ indicates an additional fungicide application at a non-specific plant stage;
Seed treatment
Seed dressing Fluxapyroxad was estimated to significantly protect an average of 0.284 t/ha barley yield (P<0.001). Growers should also consider fungicide resistance and the risk of other fungal diseases, such as smut, rhizoctonia and pythium, when considering the appropriate seed dressing.
Yield threshold and disease pressure
The yield threshold meta-analysis (results not shown) indicated, that on average across all regions, fungicide intervention did not result in significant yield protection when the crop yielded below 3.43 t/ha. As yields increased the average yield protected would also increase at the rate of 71Kg/ha for each tonne of potential yield. Actual yield thresholds for intervention may vary depending on regional conditions (i.e. disease pressure, local weather and site yield potential). For example, trials within the Albany agro-ecological zone seem to show a lower yield threshold for intervention (approx. 2.5 t/ha) compared with Esperance (approx. 3.5 t/ha) (Figure 3).
While the meta-analysis was unable to accurately define the specific interactive effects of fungicide timing, rainfall and disease pressure, the significant interaction between fungicide timing and individual trial likely includes the of role each of these factors. More specifically, agronomic factors, time of disease onset and the speed in which it can spread through multiple infection cycles will vary between location and season. Therefore, depending on individual circumstances, some fungicide strategies may provide greater yield protection than others.
The meta-analysis which included ‘disease pressure’ did not return any association between the amount of disease at Z50 in the unsprayed control (trial disease pressure) and losses in crop yield, when compared to treatments with fungicide sprays. This might be masked by the effect of high rainfall driving increased yields in combination with fostering disease pressure.
Figure 3. Relative yield (i.e. mean treatment yield - unsprayed control yield, or yield protected relative to the unsprayed control) plotted against the actual mean treatment yields. These plots illustrate potential yield thresholds for fungicide intervention. Different shaded points were used to indicate the targeted net-blotch form for each trial, NFNB and SFNB.
Conclusion
Overall, these meta-analyses support previous research on the yield impacts of net-blotch (McLean and Hollaway 2019; McLean et al., 2022; Jayasena et al., 2007). Treatment regimes incorporating two foliar fungicides were the most effective at protecting barley yield from net-blotch. Applications at around Stem extension (Z35) and early booting (Z40 – Z50) achieved the highest yield protection. The results show that shifting a two-spray program slightly earlier or later in one or both applications, was likely to be less but still effective at protecting barley yields. Fungicide applications made ‘early’ (<Z30) were the least effective at protecting yield, this might be due to the effect of incorporating a seed treatment reducing the need for early applied foliar fungicides to suppress disease pressure. In most years, delaying the first fungicide application to late stem elongation (Z35–Z39) would result in greater yield benefits than spraying earlier. Applications made following stem elongation would likely protect disease infection on the upper canopy, which is important for grain production. Fungicide applications after head emergence (Z50), on average, were capable of protecting some yield but only if the crop was not already protected with one or two fungicide applications already.
Considering this, these results also reveal greater flexibility in fungicide application timing than individual experiments, and that management actions can benefit from considering local climate and conditions. Fungicide resistance in net-blotch populations will also impact the response to fungicide programs. Net-blotch is recognised as being at high risk of developing fungicide resistance, particularly to group 7 (SDHIs). Avoiding unnecessary sprays, which don’t provide an economic benefit, and rotating active ingredients can lower the risk of resistance developing (see AFREN website).
Despite the significance of some of these findings, uncertainty remains in some of the treatments which were not represented by the same number of trials as other treatments. Additionally, some agro-ecological zones contained a greater number of trials than others (Figure 3). While a network meta-analysis does provide flexibility to compare treatments which did not co-occur in the same trial (Madden et al., 2016), the inference certainty is much lower if a treatment is not represented evenly among many of the trials. More field trial data from future research, such as GRDC investment DAQ2304-008RTX (Integrated management strategies for NFNB in low, medium, and high rainfall zones), will help refine and improve the confidence of this meta-analysis and resulting management recommendation.
References
Deadman ML and Cooke BM (1987) Effects of net blotch on growth and yield of spring barley. Annals of Applied Biology. 110:33–42.
Jayasena KW, Loughman R and Majewski J (2002) Evaluation of fungicides in control of spot-type net blotch on barley. Crop Protection. 21:63–69.
Jayasena KW, Van Burgel A, Tanaka K, Majewski J and Loughman R (2007) Yield reduction in barley in relation to spot-type net blotch. Australasian Plant Pathology. 36:429–433.
Joergensen J (1980) Comparative testing of barley seed for inoculum of Pyrenophora graminea and P. teres in greenhouse and field. Seed Science and Technology. 8:377–381.
Khan TN (1989) Effect of spot-type net blotch (Drechslera teres (Sacc.) Shoem) infection on barley yield in short season environment of northern cereal belt of Western Australia. Australian Journal of Agricultural Research. 40:745–752.
Madden LV, Piepho HP and Paul PA (2016) Statistical models and methods for network meta-analysis. Phytopathology. 106:792–806.
McLean MS and Hollaway GJ (2019) Control of net form of net blotch in barley from seed and foliar applied fungicides. Crop and Pasture Science. 70:55–60.
McLean MS, Howlett BJ and Hollaway GJ (2009) Epidemiology and control of spot form of net blotch (Pyrenophora teres f. maculata) of barley: a review. Crop and Pasture Science. 60:303–315.
McLean MS, Poole N, Santa IM and Hollaway GJ (2022) Efficacy of spot form of net blotch suppression in barley from seed, fertiliser and foliar applied fungicides. Crop Protection. 153:105865.
McLean MS, Weppler R, Howlett BJ and Hollaway GJ (2016) Spot form of net blotch suppression and yield of barley in response to fungicide application in the Wimmera region of Victoria, Australia. Australasian Plant Pathology. 45:37–43.
Van den Berg C and Rossnagel B (1991). Epidemiology of spot-type net blotch on spring barley in Saskatchewan. Phytopathology. 81:1446–1452.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support. I would also like to extend my thanks to the biometricians who supported the data collection and provided experimental summaries, Clayton Forknall, Bethany Rognoni (QDAF), all the biometricians involved at DPIRD and Kaye Basford (UQ) for her patience and guidance. I would also like to acknowledge the support of Josh Fanning from AgVic to the DAW2112-002RTX project.
Contact details
Dr Paul Melloy
The University of Queensland
8107 Plant Protection Building
The University of Queensland, Gatton Campus
Ph: 0432 601 403
Email: p.melloy@uq.edu.au
Date published
February 2024
GRDC Project Code: DAW2112-002RTX,