Retained canola seed – disease implications
Author: Steve Marcroft (Marcroft Grains Pathology, Horsham, VIC), Angela van de Wouw (University of Melbourne, VIC) Kurt Lindbeck (NSWDPI, Wagga Wagga, NSW) | Date: 20 Feb 2024
Take home messages:
- Retained seed may be infected with blackleg, if pod infection occurred in your 2023 crop. Either do not retain the seed for sowing or treat the seed with a registered blackleg fungicide.
- Be aware that retained seed may have a lower blackleg rating than stated in the GRDC blackleg management guide. Monitor current crops in the spring for blackleg severity to determine if your retained seed crop has lost resistance.
- In 2023, cultivars had varying blackleg severity due to differences in environment (proximity to canola stubble and rainfall) and regions where individual resistance genes are being overcome.
- Sclerotinia in southern Victoria is very sporadic, individual crops can have very large differences in disease severity.
- Fungicide applications against Sclerotinia were very effective but not all crops had enough disease to warrant control.
- The Blackleg Management App has been developed to provide growers and advisers with an interactive interface to explore the economic outcomes of different blackleg management strategies and their relative importance.
Retained canola seed – disease threats to manage
Seed quality
Canola pods are often colonised by fungi prior to harvest. Many of these fungi are not pathogenic against canola, however both blackleg and Alternaria species can colonise pods and are pathogenic. GRDC is currently investing in Alternaria research to determine the geographic spread and severity of the disease, whether Alternaria causes damage to seed that is retained for sowing, and potential yield losses associated with the disease. What is currently known is that Alternaria will cause pods to shatter causing subsequent yield loss; in very severe situations, yield losses of up to 20% have been confirmed. The current GRDC project will report on work investigating if Alternaria infected pods lead to infected seed, and if infected seed results in seedling blight. The project will also investigate if commercially available seed treatments will reduce seedling blight caused by Alternaria. Current recommendations are to avoid retaining/sowing seed from paddocks that have Alternaria pod infection. Alternaria infection is associated with wet conditions post-flowering.
Blackleg is also able to form lesions on canola pods. Blackleg, like Alternaria, will also cause pod shatter. However, blackleg will penetrate the pod to directly infect seeds below the lesion. If blackleg-infected sown is sown, seedling blight will cause seedling death. Infected seed may also be small and shrivelled, reducing the seed germination percentage and reducing seedling vigour. It is highly recommended to avoid retaining canola seed from crops with severe blackleg pod infection. Seed treatments designed to control blackleg will also protect against blackleg seedling blight, so applying a registered blackleg seed treatment fungicide is highly recommended.
Sclerotinia infects canola stems and branches, it does not directly impact seed. Sclerotinia, however, survives by forming sclerotia which are very hard and robust compact mycelia; it is these sclerotia which can contaminate canola seed. Therefore, if you retain seed from a Sclerotinia-impacted crop, be sure that the seed does not contain sclerotia.
Cultivar blackleg rating
There are two types of blackleg resistance genes in Australian canola cultivars: major and quantitative resistance genes. Major resistance genes stop the fungus from infecting the plant, which results in complete protection against the blackleg pathogen. This is evidenced in the field by a lack of leaf lesions and crown canker. A cultivar can have none, one or multiple major resistance genes. In Australia, all commercial canola cultivars are classified into resistance groups which describe the major genes present in the cultivar. For example, Group A cultivars have a single major resistance gene, Group ABDF cultivars have four major resistance genes. Blackleg is rapidly able to change, and major gene resistance imposes strong selection for only those isolates of blackleg able to infect the plant. Therefore, major resistance genes can be overcome quickly in the field, so cultivars dependent on major resistance genes tend to become more susceptible over time, sometimes becoming completely ineffective in as little as 3 years.
Quantitative resistance (sometimes called minor gene, adult plant or crown canker resistance) reduces the severity of disease, including reduced crown canker, leaf lesions and upper canopy infection. As the name suggests, each gene provides a minor level of disease resistance. However, the combined effect of a number of minor genes in the same cultivar can create very high levels of resistance. Similar to major gene resistance, quantitative resistance can be overcome by the blackleg fungus. However, as there are many genes involved with quantitative resistance, the breakdown in resistance may take longer and is less catastrophic; that is, it is more of an erosion of resistance over time.
Retained seed is generally more prone to the loss of blackleg resistance, as open pollenated cultivars may be retained for more years compared to hybrid cultivars. Seed companies monitor and are aware of loss of resistance to blackleg, however as they know that it will occur, they are constantly breeding to improve genetic blackleg resistance. Therefore, when a commercial hybrid begins to get more disease than expected, the seed companies will stop selling the hybrid and replace it with a new hybrid that has yet to be overcome by the blackleg fungus. However, open pollinated cultivars that are retained by growers may stay in the system for many years, so this means that the fungus has more time to overcome the cultivar’s resistance. There are current open pollinated retained cultivars that started life with an MR rating before falling to an MRMS after 3 years and are now rated MS. Although blackleg resistance in most cultivars is highly likely to fall over time, the rate and amount of decline is also dependent on the popularity of the cultivars and other cultivars with similar or the same blackleg resistance. That is, if a cultivar is sown over a large area in tight rotation, there will be a higher density of spores attacking the crop and the probability of the resistance being overcome will increase. If a cultivar is only sown on a small area, its resistance may last for a longer time period.
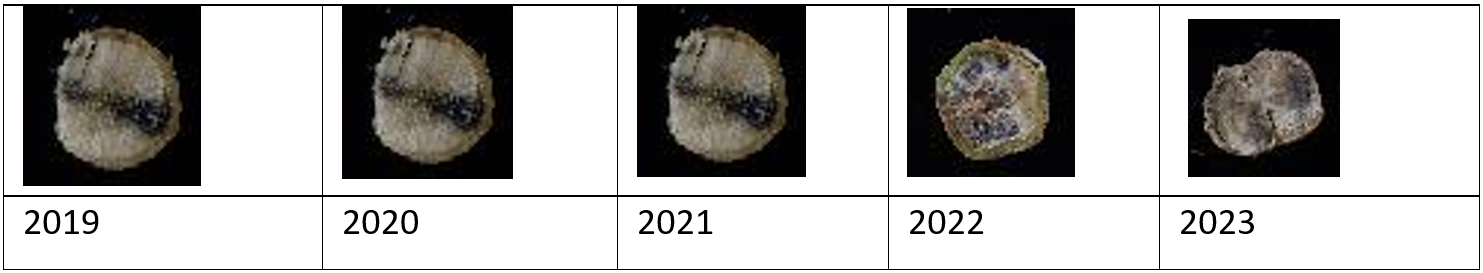
Figure 1. Images of crown canker from the same cultivar over time. In the past 2 years, the cultivar should have had fungicide protection or been replaced with a more resistant cultivar.
To reduce the effect of blackleg resistance being overcome or resistance erosion occurring, it is critical to monitor your crops for blackleg disease. If your crops consistently have, on average, less than 40% internal infection of the crown, they will have low yield losses associated with blackleg. Higher levels of infection means that your crop management should be updated. You can increase the distance between your canola crop and the previous year’s canola stubble, protect the crop with seed treatment and foliar fungicides, or change cultivars to a different resistance group.
If your cultivar started life with effective major gene resistance, for example, it has the new resistance gene Group H, it should be immune to blackleg and therefore not get leaf lesions. If you monitor your crop and it has leaf lesions, then it is highly likely that the Group H resistance gene is being overcome. In this circumstance, you should consider a foliar fungicide application.
How to monitor your crop for blackleg disease
Plants should be randomly selected; lodged plants should be inspected for crown cankers, other plants should be severed with a pair of secateurs at the junction between the roots and the stem. Look for the percentage of discolouration. Plants with 40% or greater discolouration (Figure 2) are highly likely to have yield losses.
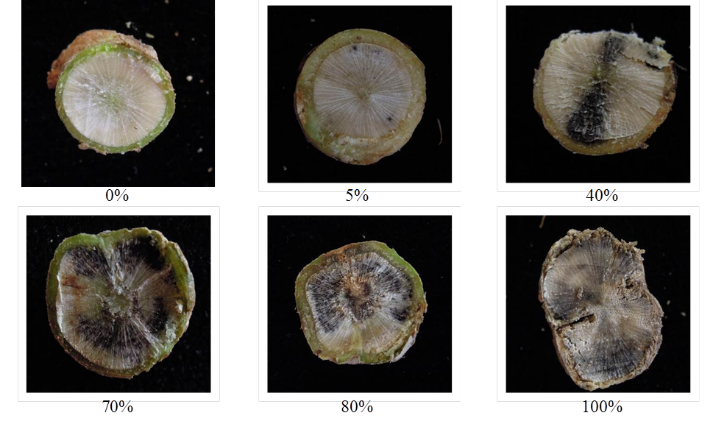
Figure 2. Canola crowns cut with secateurs to enable plants to be scored for blackleg infection.
Blackleg severity in 2023
In 2023, blackleg levels were considered relatively normal. Most crops were sown in late April/early May, resulting in a typical flowering window during mid-August. A wet winter period led to crown canker but a drier than normal spring and flowering time in mid-August (in 2022, flowering commenced in July) meant that upper canopy blackleg was not severe.
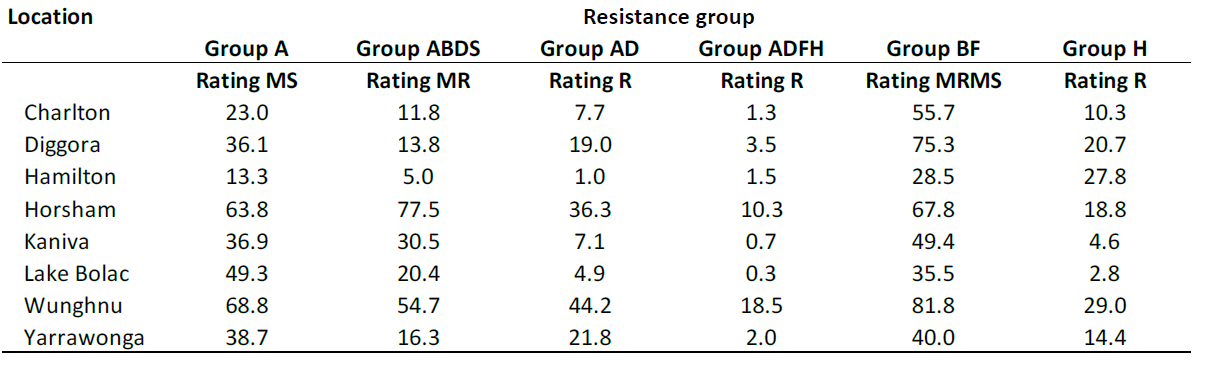
Table 1 shows the average percentage crown canker (internal infection as per Figure 2) for the blackleg monitoring sites. The data show that blackleg severity (no fungicide protection) ranged from no disease to over 80% infection, dependent on the site and cultivar screened. Differences in cultivars within the same site (same disease pressure) indicates the extreme difference cultivar blackleg resistance can have on disease severity and subsequent yield losses. The data also demonstrate why it is crucial to monitor crops and change practices when warranted.
The Group A cultivar is dependent on quantitative resistance. The difference in disease between sites for this cultivar is due to difference in disease pressure (distance to canola stubble and rainfall), but is also an indicator that, in some regions, this quantitative resistance has been overcome by the pathogen. The data also show some cultivars, even when rated R, still have high disease in individual locations, which indicates that resistance is being overcome and cultivars now need replacing or protection from fungicides.
Table 1: 2023 blackleg monitoring sites, data is the average crown (canker) infection.
2023 Sclerotinia in southern Victoria
Sclerotinia stem rot can cause substantial yield loss in canola, but outbreaks are sporadic and heavily dependent on weather conditions during the season. Sclerotinia has a complex disease cycle that has several key stages that must have favourable weather conditions for the disease to progress. In the life cycle, the sclerotia (survival structures) in the soil mature in wet conditions and form apothecia (fruiting bodies) which release spores into the air. These spores land on and infect the flowers. As the flowers senesce, the petals drop off and get caught on leaves, in leaf junctions and in the branches. If weather conditions are favourable, the Sclerotinia fungus will grow from the petals into the stem or branch, causing the infection. Typically, it is wet weather during spring when the canola is flowering that increases the chance of infection occurring, especially if the canopy stays wet for more than 48 hours.
The GRDC Sclerotinia investment is investigating the severity and implication of Sclerotinia in western Victoria. The data from 26 paddocks over two years has shown that Sclerotinia infection is very variable between years, seasons and individual paddocks (see Table 2). However, the fungicide applications were very effective at controlling disease, albeit many of the paddocks had low levels of infected plants and therefore may not have benefited from a fungicide application. Individual paddocks may have had a large economic return from fungicide application in both years and from both regions. The most severe infection in the paddock at west Willaura in 2022 may have had up to 20% yield loss.
The aim of this research program is to determine if Sclerotinia infection triggers can be determined from weather conditions. In southern NSW, researchers have found that, during the crop bloom stage, periods of relative humidity that exceed 95% for more than 48 hours and where petal infection exceeds 50% at the same time will cause Sclerotinia infection to occur. However, Sclerotinia infection is rarely as severe in western Victoria, even though extended periods of high relative humidity are regularly experienced.
This project has measured infection events (48h at >95% RH and >50% petal infection) over two years and two different regions in western Victoria. The data have shown a strong relationship between spring rainfall and infection; in 2022, there was five times more disease compared to 2023 (wet compared to dry spring). However, there is considerable variation between individual paddocks. The data also appears to show that infection events do not correlate with Sclerotinia symptoms in individual crops (see Table 2). This finding supports anecdotal agronomist knowledge that conditions in western Victoria are not as conducive for Sclerotinia development, regardless of the known infection events.
Although Sclerotinia infection is not as severe in western Victoria, the data have still shown that 66% of paddocks had greater than 10% of plant infection in 2022 (four out of six paddocks) and 8% of paddocks had greater than 10% of plant infection in 2023 (two out of 20 paddocks). This data indicates that growers and agronomists cannot be complacent and must still manage Sclerotinia, even if it is less likely to occur than in other Australian regions.
The triggers for infection events in western Victoria remain unknown and the data from this project will be provided to NSWDPI for further analysis. Experiments will continue for the next two years.
Table 2: 2022 and 2023 Sclerotinia stem rot infection for both the Western district and the Wimmera, Victoria.
Year | Location | 1st flower | Number of known infection events | % plants with stem rot | |
|---|---|---|---|---|---|
Untreated | Fungicide applied at 30% bloom | ||||
2022 | NW Willaura | 12-Aug | 2 | 3 | 0 |
2022 | W Willaura | 10-Aug | 2 | 44 | 6 |
2022 | Lake Bolac | 15-Aug | 2 | 16 | 12 |
2022 | Westmere | 7-Aug | 1 | 10 | 4 |
2022 | W Streatham | 16-Aug | 4 | 8 | 5 |
2022 | N Streatham | 3-Aug | 0 | 14 | 7 |
2023 | Lake Bolac | 7-Aug | 0 | 2 | 0 |
2023 | W Willaura | 3-Aug | 0 | 3 | 0 |
2023 | N Willaura | 12-Aug | 0 | 7 | 5 |
2023 | N Mortlake | 17-Aug | 0 | 1 | 0 |
2023 | Lake Goldsmith | 15-Aug | 0 | 0 | 1 |
2023 | Woorndoo | 1-Aug | 0 | 2 | 0 |
2023 | Langi Logan | 14-Aug | 0 | 8 | 1 |
2023 | Streatham | 1-Aug | 0 | 10 | 2 |
2023 | N Willaura | 10-Aug | 0 | 3 | 0 |
2023 | N Streatham | 10-Aug | 0 | 3 | 5 |
2023 | E Dimboola | 4-Sep | 0 | 0 | 0 |
2023 | S Kewell | 29-Aug | 0 | 0 | 0 |
2023 | N Minyip | 8-Aug | 0 | 1 | 0 |
2023 | NE Minyip | 1-Sep | 0 | 0 | 0 |
2023 | N Dimboola | 2-Aug | 0 | 6 | 0 |
2023 | W Dimboola | 2-Aug | 0 | 3 | 2 |
2023 | Dimboola | 5-Aug | 0 | 12 | 0 |
2023 | Minyip | 13-Aug | 0 | 3 | 1 |
2023 | N Minyip | 13-Aug | 0 | 0 | 0 |
2023 | Tarranyurk | 31-Jul | 0 | 2 | 1 |
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial co-operation and the support of the GRDC, the authors would like to thank them for their continued support.
Useful resources
The National Variety Trials Program
Contact details
Steve Marcroft
Marcroft Grains Pathology
Grains Innovation Park, Natimuk Rd, Horsham VIC 3400
(03) 5381 2294
0409 978 941
Steve@grainspathology.com.au
GRDC Project Code: MGP2307-001RTX, MGP24-0-002RTX, MGP2402-002RTX, DPI2206-023RTX,
Was this page helpful?
YOUR FEEDBACK
