Yield potential of synthetic auxin herbicide tolerant field pea
Yield potential of synthetic auxin herbicide tolerant field pea
Author: Simon Michelmore, Timothy Sutton (Waite Research Institute, School of Agriculture, Food and Wine, The University of Adelaide), Philip Brewer, Matthew Tucker (Waite Research Institute, School of Agriculture, Food and Wine, The University of Adelaide) | Date: 06 Feb 2024
Take home messages
- New pulse varieties with improved tolerance to synthetic auxin herbicides are being trialled.
- Herbicide tolerance traits don’t have to come with a big yield penalty.
- A 27% increase in grain yield is possible through a single gene regulating plant architecture.
- Plant molecular biology PhDs can have applied outcomes.
Background
Herbicide tolerant crop varieties provide novel weed control options, increased crop safety, and offer more flexible crop rotations. Weed control is particularly important in the pulse phase because pulses have poor early crop competition, few registered herbicide options, and are sensitive to soil residues of herbicides used in previous seasons. Many herbicide tolerant crops have been developed, but these traits tend to come at the cost of grain yield potential. Triazine tolerant canola has an inherent yield penalty of 20–30% (Robertson et al. 2002) and a similar penalty is seen in metribuzin tolerant lentils (McMurray et al. 2021). While this can be an acceptable trade off in some situations, it is important to understand and minimise yield penalties where possible.
Molecular genetics research provides an opportunity to understand the molecular and physiological basis for key agronomic traits. For example, understanding the effects of herbicide tolerance traits on plant development may reveal strategies to overcome associated yield penalties. This PhD project sought to characterise and understand the effects on plant development and grain yield of new synthetic auxin herbicide tolerance traits developed by SARDI and UoA (GRDC projects DAS00131 and UOA2007-010RTX).
Synthetic auxin herbicides like clopyralid (Lontrel®) act through the plant’s hormone signalling pathways, where they mimic the phytohormone auxin, leading to an overactivation of auxin responses and plant death (Todd et al. 2020). Target-site tolerance is possible through mutations in an auxin receptor gene; however, if the mutation alters the function of the gene too severely, there are potential implications for the normal developmental processes regulated by this hormone signalling pathway.
As part of the previous GRDC-funded projects, ethyl methanesulfonate (EMS) mutagenised field pea (Pisum sativum) lines with improved clopyralid tolerance were identified through bulk screening of a PBA Wharton-derived mutant population. Through those projects and this PhD research, 14 unique tolerance mutations have been identified in the Auxin Signalling F-box Protein 4/5 (AFB4/5) auxin receptor gene (in-press; this work). Different mutants show varying degrees of altered plant architecture, delayed phenology, and reduced grain size in greenhouse and field conditions.
Here, we report that the seed size of a herbicide-tolerant mutant can be completely rescued by a second mutation in a gene that acts downstream of auxin signalling. Furthermore, while most clopyralid tolerant mutants have an inherent yield and seed size trade-off, one mutant line substantially outperformed PBA Wharton, with up to 21% higher average grain weight and 27% higher yield in optimal field conditions.
Method
Field trials were conducted in the South Australian mid-north region (Table 1). A preliminary assessment of 90 mutant lines (unreplicated) alongside check rows of PBA Wharton was conducted in 2020 and observations compared with a similar trial conducted simultaneously by the National Field Pea Breeding program (Agriculture Victoria Research). Short rows were hand harvested to ensure seed purity.
For trials in the 2022 and 2023 seasons, experimental plots (12m x 1.5m) were arranged in a randomised complete block design with four replicates. Seeding rate treatments were based on a target plant density of 45 plants/m2.
Controlled environment experiments were performed in growth rooms at the University of Adelaide, Waite Campus. Seeds were sown in 20cm diameter 4.5L pots of BioGro potting soil (90% composted pine bark + 10% river sand) and growth conditions were maintained at 24/15°C day/night, 14-hour day length and maximum light intensity of 700µmol/m2/s. The smxl7-1 mutant line (Kerr et al. 2021) was kindly provided by Dr Elizabeth Dun (University of Queensland) and double mutants were generated by crossing with the afb4/5-8 null mutant.
Statistical analyses were performed using the ASREML package (Butler 2022) in R Statistical Software (v4.2.0; R Core Team 2022), and GraphPad Prism version 9.0.0 for Windows (GraphPad Software, Boston, Massachusetts USA,)
Table 1: Field trial sites. Locations are approximate.
Turretfield 2020 | Mallala 2022 | Kapunda 2022 | Kapunda 2023 | |
|---|---|---|---|---|
Location (latitude, longitude) | -34.549, 138.795 | -34.475, 138.563 | -34.389, 138.889 | |
Mean annual rainfall (mm) Growing season (Apr–Oct) | 371.6 (274.9) | 381.4 (256.1) | 491 (335.2) | |
Actual rainfall Growing season (Apr–Oct) | 336.8 (284.4) | 533.8 (353.6) | 689.8 (510.3) | 407.2 (272.7) |
Results and discussion
Preliminary field assessment
Plants carrying predicted null (complete loss of function) mutations in the auxin receptor/herbicide target-site gene consistently showed severe growth defects including reduced plant height, increased shoot branching, delayed phenology and reduced seed size, while those with substitution mutations causing a small change in the predicted protein sequence had a range of intermediate phenotypes that varied between the control and null lines (Figure 1A). Two clopyralid-tolerant mutant lines with growth habit and thousand grain weight similar to PBA Wharton were selected in consultation with the breeder for further evaluation (Figure 1B). Line 17KAHCL038 had only minor changes in plant height and phenology (data not shown) but 16% lower average seed weight (p<0.01, Student’s independent T-test). Line 17KAHCL050 had slightly reduced plant height and delayed phenology but seed size was not reduced (p=0.36).
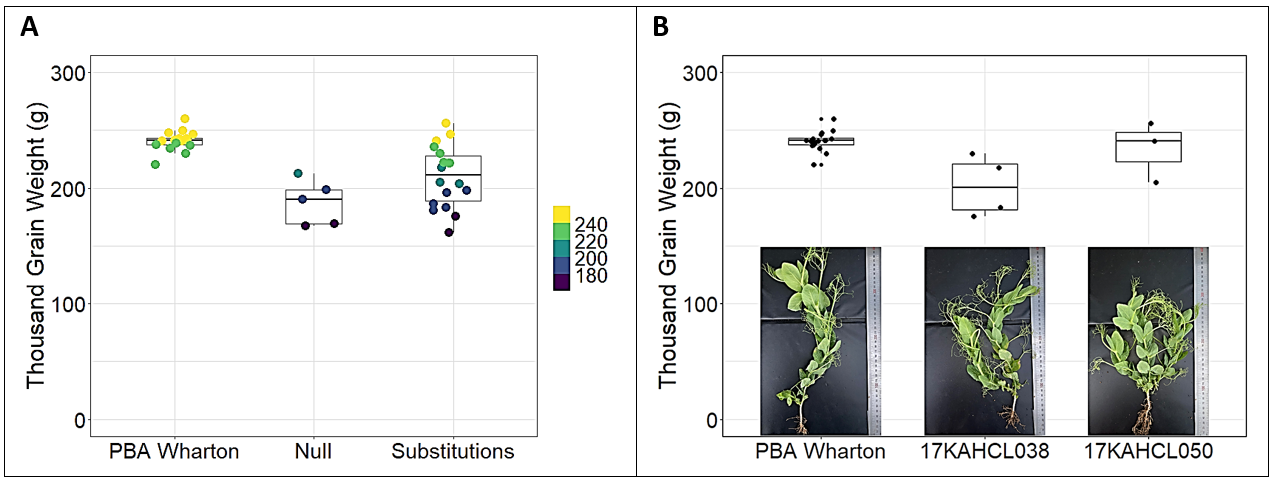
Figure 1. Preliminary field assessment reveals reduced seed size in clopyralid tolerant field pea mutants. Five null and 18 substitution mutant lines showed contrasting effects on thousand grain weight (A). Two mutations of interest for breeding were replicated within the 2020 field trial (B).
2022 field trials
Field sites were selected to represent low-medium rainfall (Mallala; 256mm mean growing season rainfall) and medium-high rainfall (Kapunda; 335mm mean growing season rainfall) zones, based on a growing season of April–October. There was unusually high rainfall in 2022, with annual rainfall in the 95th percentile at both sites, resulting in a delayed harvest and extended growing season. Actual growing season rainfall (April–November) was 460.4mm at Mallala and 645.5mm at Kapunda (Australian Government Bureau of Meteorology).
At Mallala, 17KAHCL038 showed an 18% reduction in grain yield compared to PBA Wharton at the 0.5x seeding rate (p=0.04) but no significant difference at the 1x seeding rate (p=0.89). In contrast, 17KAHCL050 showed a substantial yield improvement of 22% (p=0.01) and 27.5% (p=0.003) at the 0.5x and 1x seeding rates, respectively (Figure 2A). These yield differences were driven partly by seed size, with 17KAHCL038 showing a 7.6% reduction in hundred seed weight (100SW) compared to PBA Wharton at the lower seeding rate, while 17KAHCL050 had a 17% and 25% increase in 100SW at the reduced and standard seeding rates, respectively (Figure 2B).
Due to poor emergence, waterlogging, and weed pressure, the Kapunda site performed poorly and had large variances in grain yield between replicates. Mean grain yields followed the same pattern as Mallala, however none of these differences met the threshold for statistical significance (Figure 2C).
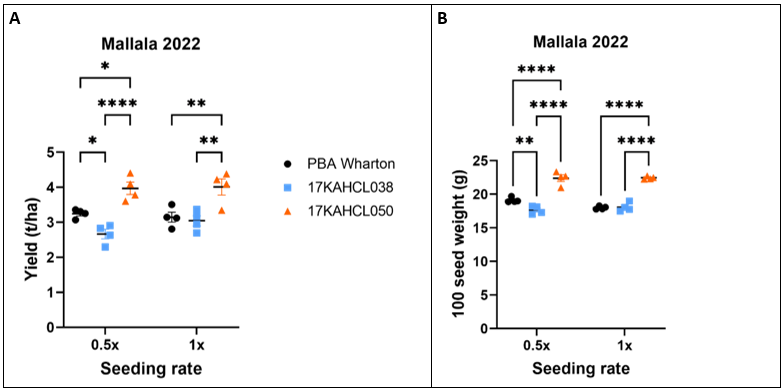
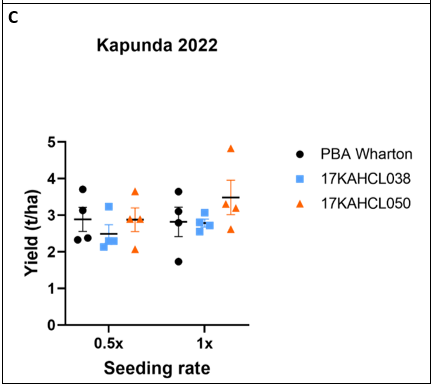
Figure 2. Performance of two herbicide tolerant field pea lines compared to the susceptible variety PBA Wharton in field trials at Mallala (A, B) and Kapunda (C) in 2022. No significant effect of seeding rate was detected in a 2-way ANOVA. Genotypic differences marked with asterisks are significant at p<0.05 (*), p<0.01 (**), p<0.005 (***) and p<0.0001 (****) (Tukey’s multiple comparisons test).
2023 field trials
To test whether the increased yield of 17KAHCL050 could be replicated in more limiting field conditions, two times of sowing (TOS) were included, ~5 weeks apart with the aim to compare normal conditions to a shortened growing season. The 2023 year was drier than average, with growing season rainfall (April–October) totalling 272.7mm, compared to the long-term mean of 335mm. A hot, dry spring resulted in a harsh finish to the season and harvest was 5 weeks earlier than in 2022.
Line 17KAHCL050 performed well at the 1x seeding rate at TOS1, with no significant reduction in mean grain yield (p=0.17), however a significant yield penalty of 10% was observed at the reduced seeding rate (p=0.05; Figure 3A). TOS2 yield was lower for both genotypes. Line 17KAHCL050 showed a 12% yield penalty (p=0.0006) at the 1x seeding rate and 11% at the 0.5x rate (p=0.006; Figure 3B). The average seed weight of the herbicide tolerant mutant was ~7–10% larger than that of PBA WhartonA in all treatments (Figure 3C-D).
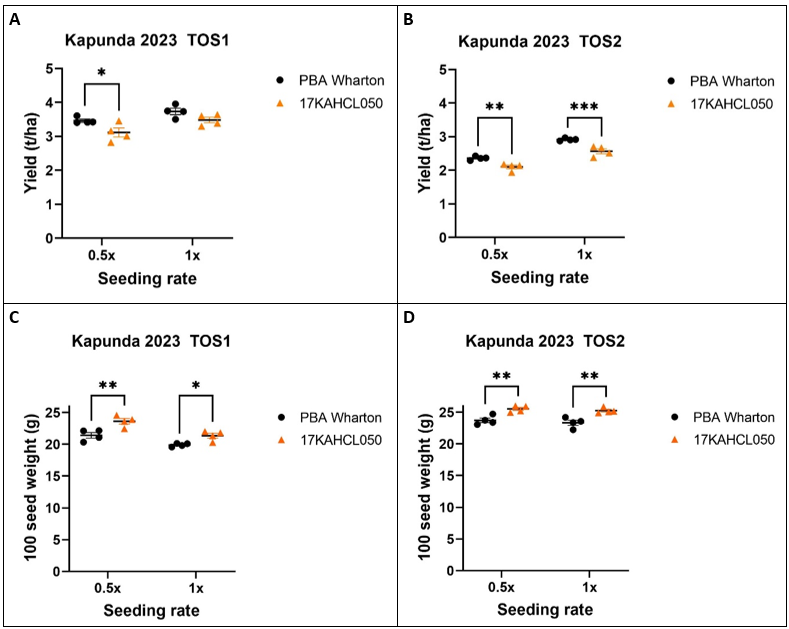
Figure 3. A yield penalty associated with the 17KAHCL050 clopyralid herbicide tolerance trait is apparent with delayed sowing and at reduced seeding rates. Grain yield (A, B) and 100 seed weight (C, D) were measured for two times of sowing (TOS). Seeding rates targeted 0.5x and 1x standard plant density of 45 plants/m2. Differences marked with asterisks are significant at p<0.05(*), p<0.01 (**), and p<0.005 (***) (2-way ANOVA and Bonferroni’s multiple comparisons test).
SMXL7 regulates seed size
Auxin regulates diverse processes in plant growth and development through a complex network of receptors, co-receptors and auxin response factors controlling expression of hundreds of genes. In shoot branching regulation, strigolactones are another hormone that act downstream of auxin to repress lateral bud growth. In garden pea, Kerr et al. (2021) described a mutant of SUPPRESSOR OF MAX2-LIKE 7 (SMXL7) which restores branching regulation in strigolactone signalling mutants. They demonstrated that the smxl7-1 mutation partially rescues branching regulation and plant height in an afb4/5-1 auxin receptor mutant. To test if the reduced seed size of the 17KAHCL065 null mutant (afb4/5-8) is due to reduced strigolactone response via SMXL7, we crossed the herbicide tolerant line with the smxl7-1 mutant and identified single and double mutants in the F3 generation.
All afb4/5-8 single mutants were dwarfed, highly branched and had a 35% decrease in average seed weight compared to wildtype F3 lines. The afb4/5-8 smxl7-1 double mutants had partially restored shoot architecture, as expected, and seed size was completely restored (Figure 4). This finding suggests that SMXL7 plays an important role in seed development downstream of auxin signalling and shows that smxl7 mutants may be able to minimise yield and grain quality trade-offs when breeding for synthetic auxin herbicide tolerance.
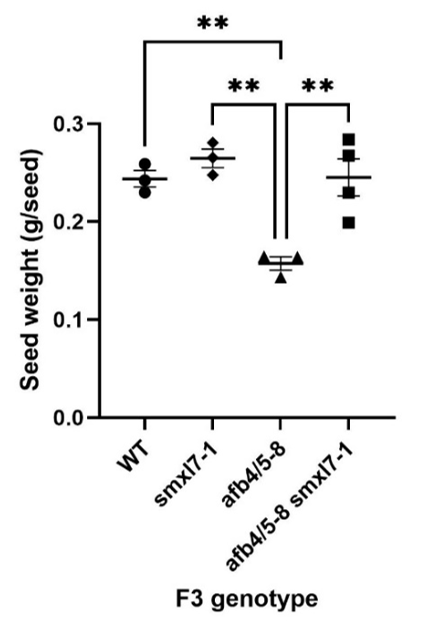
Figure 4. Reduced seed weight of the afb4/5-8 null mutant is rescued by smxl7-1. Data points are average seed weights from individual F3 plants grown in controlled environment. Differences marked with asterisks (**) are significant at p<0.01 (1-way ANOVA and Tukey’s Multiple Comparisons Test).
Conclusion
A yield penalty is associated with some synthetic auxin herbicide tolerance mutations in field pea, but the magnitude of the penalty varies between mutation events and with environmental conditions. We demonstrated that the reduced seed size observed in some clopyralid tolerant mutants (namely, 17KAHCL065, afb4/5-8) can be rescued by a mutation in another single gene in the auxin-strigolactone hormone signalling pathway (namely, smxl7-1). Additionally, we identified a mutant line with a 27% increase in grain yield compared to PBA Wharton in favourable (long-season) field conditions, but a small (0–12%) yield penalty in more limiting conditions.
Through exploring novel large-scale genetic diversity and understanding the molecular biology underpinning key agronomic traits, we showed that it is possible to develop herbicide tolerant crops that offer valuable weed control options while minimising the trade-off in crop productivity. Furthermore, we showed that there is an exciting opportunity to achieve a step-change in yield potential through targeting plant architecture in grain legumes.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support. This research is supported by an Australian Government Research Training Program (RTP) Scholarship.
References
Butler D (2022) _asreml: Fits the Linear Mixed Model_. R package version 4.1.0.176
Kerr SC, Patil SB, de Saint Germain A, Pillot J-P, Saffar J, Ligerot Y, Aubert G, Citerne S, Bellec Y, Dun EA, Beveridge CA, Rameau C (2021) Integration of the SMXL/D53 strigolactone signalling repressors in the model of shoot branching regulation in Pisum sativum. The Plant Journal 107(6), 1756–1770.
McMurray LS, Preston C, Vandenberg A, Munoz-Santa I, Mao D, Bett KE, Michelmore S, Paull JG (2021) Paternal leakage inheritance and a fitness cost are associated with the chloroplastic psbA gene controlled metribuzin tolerance in lentil (Lens culinaris). Euphytica 217, 103.
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL
Robertson MJ, Holland JF, Cawley S, Potter TD, Burton W, Walton GH, Thomas G (2002) Growth and yield differences between triazine-tolerant and non-triazine-tolerant cultivars of canola. Australian Journal of Agricultural Research 53, 643–651.
Todd OE, Figueiredo MRA, Morran S, Soni N, Preston C, Kubeš MF, Napier R, Gaines TA (2020) Synthetic auxin herbicides: finding the lock and key to weed resistance. Plant Science 300, 110631.
Contact details
Simon Michelmore
Waite Research Institute, The University of Adelaide
Simon.michelmore@adelaide.edu.au
@SimonsPea_hD
GRDC Project Code: UOA2006-009RSX,
