Incidence of decreased sensitivity to fungicides in barley net blotch in Queensland
Incidence of decreased sensitivity to fungicides in barley net blotch in Queensland
Author: Noel L. Knight (USQ/Curtin University), Lisle Snyman (QDAF), Anke Martin (USQ), Levente Kiss (USQ), Francisco Lopez-Ruiz (Curtin University) | Date: 31 Jul 2024
Take home message
- Decreased sensitivity to fungicides is present in net blotch of barley in Queensland
- Frequencies of decreased sensitivity were 34% for Group 3 fungicides and 6% for Group 7 fungicides in a regional population
- Fungicide management strategies should rotate or use mixed modes of action
- Decisions can be informed by testing field samples for fungicide sensitivity
Aim
Determine the presence and frequency of decreased sensitivity to Group 3 and Group 7 fungicides in net blotch affecting barley fields in Queensland.
Introduction
Chemical control of barley net blotch relies on three major classes of fungicides, demethylation inhibitors (DMI; FRAC Group 3), succinate dehydrogenase inhibitors (SDHI; FRAC Group 7) and quinone outside inhibitors (QoI; FRAC Group 11). Decreased sensitivity to fungicides in the net form (Pyrenophora teres f. teres) and spot form (P. teres f. maculata) net blotch pathogens has become an important disease management issue in Australia. Decreased sensitivity in the field may be characterised by a loss of efficacy of fungicide treatments, while in the laboratory it involves growing fungal isolates across increasing fungicide concentrations, with responses ranging from sensitive to reduced sensitive to resistant.
Reduced sensitivity and resistance to Group 3 fungicides in net blotch has been reported in Western Australia from 2016 (Mair et al., 2016; Mair et al., 2020), while reduced sensitivity and resistance to Group 7 fungicides was first detected in 2019 in South Australia (Mair et al., 2023). Reduced sensitivity to Group 11 fungicides was also first detected in South Australia in 2022. No monitoring of fungicide sensitivity in net blotch has been reported for Queensland.
DNA markers associated with decreased sensitivity to each fungicide have been identified, and tests are available to specifically detect these markers in disease samples from fields (Knight et al., 2021; Knight et al., 2024). These markers encompass both reduced sensitive and resistant growth responses. This approach avoids time-consuming growth tests and can be applied to bulk disease samples to report the frequency of decreased sensitivity within a field (Knight et al., 2022).
This study used a DNA marker detection approach to investigate net blotch disease from ten barley fields in southern Queensland and determine the frequency of decreased fungicide sensitivity. The results from this study inform Queensland growers of the potential risk of fungicide resistance and aid in the application of improved strategies for disease and fungicide management.
Method
Net blotch field sampling
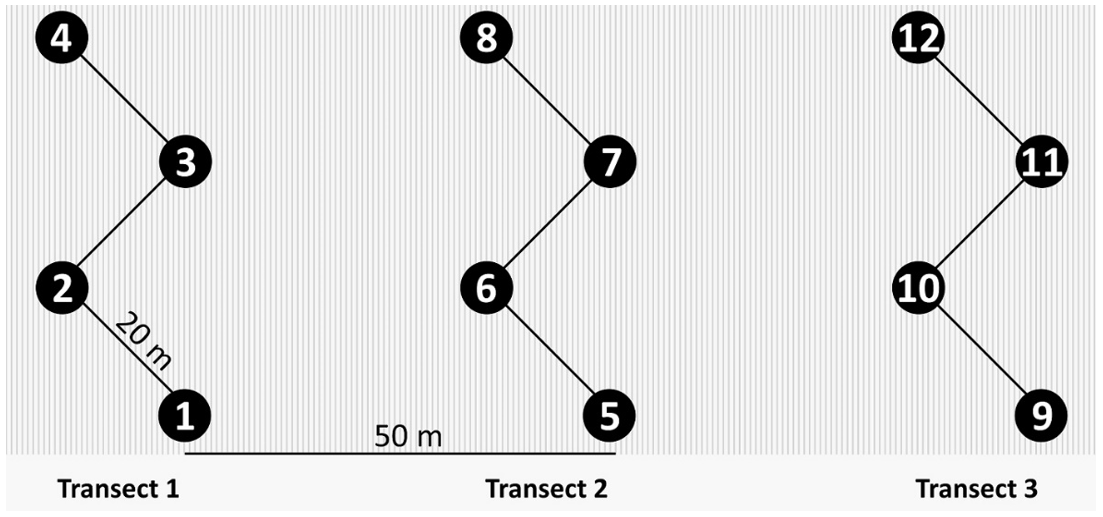
Ten barley fields across southern Queensland were sampled for net blotch disease (Figure 1). The sampling strategy consisted of leaf collection at four points across three zigzag transects in each field (Figure 2). A minimum of 10 symptomatic leaves were collected at each of the 12 sampling points in each field, preferentially targeting the upper three leaves. Sampling of leaves occurred at early to late grain fill in October to November 2022. Samples were dried at room temperature.
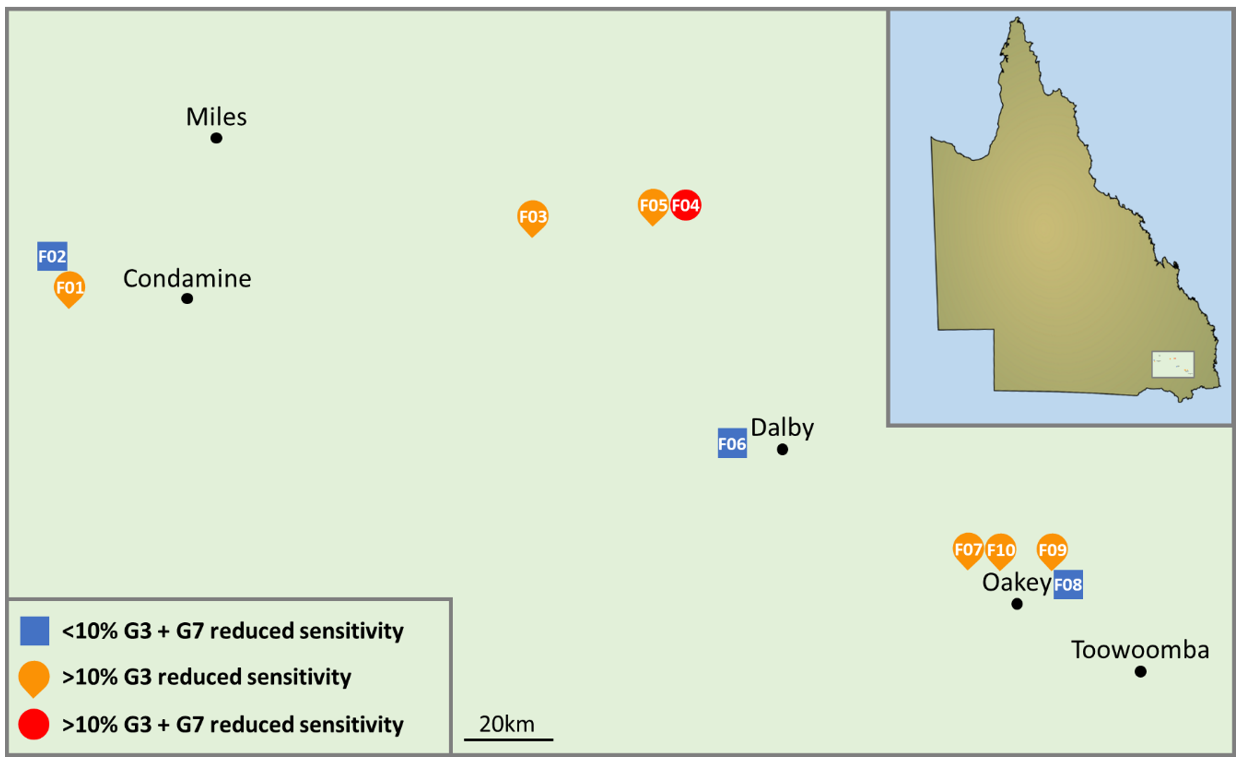
Figure 2. Sampling strategy applied to each field to collect net blotch affected leaves. A minimum of 10 diseased leaves were collected at each of the 12 points across three zigzag transects.
Determining frequencies of decreased fungicide sensitivity
For each field, a single lesion was taken from five leaves at each sampling point. These lesions were pooled across the 12 sampling points, resulting in 60 lesions per field. Tissues were dried at 65°C overnight, ground to a powder and DNA extracted from a 20mg sub-sample using a Wizard Genomic DNA Extraction Kit (Promega, NSW, Australia).
Each sample was tested in a QX200 Droplet Digital PCR System (Bio-Rad, California, United Sates). Initial DNA tests detected and quantified the net form or spot form net blotch pathogens. Each sample was then assessed for the presence of DNA markers associated with sensitivity, reduced sensitivity or resistance to Group 3 and Group 7 fungicides. Each PCR reaction was performed in a 22μL volume containing 11μL ddPCR Supermix for Probes (no dUTP) (Bio-Rad) and 5μL of DNA template. Following droplet generation and PCR, droplets were read and analysed in the QX Manager software (Bio-Rad). Results are reported as number of copies of each target in a reaction.
Results
Disease presence
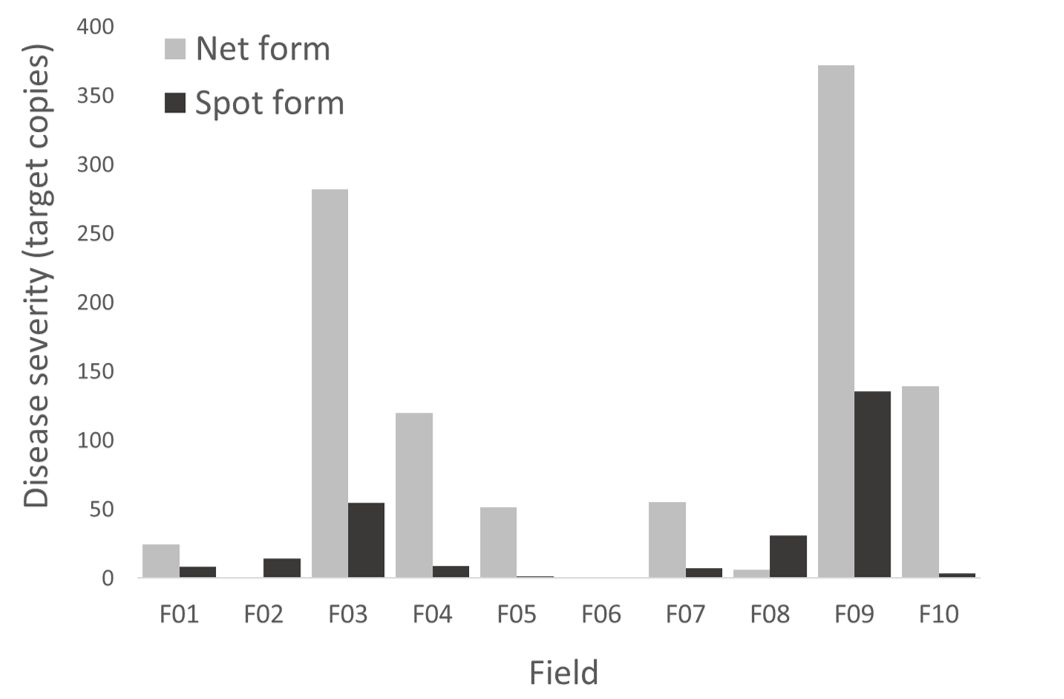
Net form net blotch was the most prevalent disease in most fields, with spot form net blotch commonly occurring at lower quantities (Figure 3). Disease severity ranged from very low to severe among the ten fields.
Figure 3. Relative severity of net form and spot form net blotch in 10 barley fields sampled in 2022. Severity was estimated based on the amount of pathogen DNA marker (target copies) present in leaf lesions.
Frequencies of decreased fungicide sensitivity
Among field populations, the frequency of markers associated with decreased sensitivity to Group 3 fungicides ranged from 0 to 68%, while markers associated with decreased sensitivity to Group 7 fungicides ranged from 0 to 13% (Table 1).
Across the entire regional collection, the frequency of decreased sensitivity to Group 3 fungicides was 34%, while the frequency of decreased sensitivity to Group 7 fungicides was 6%.
Fungicide applications varied among fields; however no strategy was linked to differences in the frequency of decreased sensitivity. Interestingly, field F08 had a long history of no fungicide use and had low net blotch severity, however decreased sensitivity markers for Group 3 fungicides were detected at 7%.
Fields F07 and F10 were the same location sampled at two timepoints, with a fungicide application in-between. This aligned with an increase in the frequency of decreased sensitivity to Group 3 fungicides from 25% to 70%.
Table 1. Frequencies of DNA markers associated with decreased sensitivity to Group 3 and Group 7 fungicides in DNA extracted from ten field samples of net blotch. The in-season fungicide applications are indicated by FRAC number1.
Field | Decreased sensitivity (%) | In-season applications | |||
|---|---|---|---|---|---|
Group 3 | Group 7 | Seed/in-furrow | Foliar | ||
F01 | 21 | 0 | 3 + 4 + 7 | 3 + 11 | |
F02 | 0 | 0 | - | 3 + 11 | |
F03 | 10 | 1 | 3 + 4 + 7 | 3 + 11 | |
F04 | 27 | 13 | 3 + 4 + 7 | 3 + 11 | |
F05 | 15 | 2 | 3 + 4 + 7 | 3 + 11 | |
F06 | 0 | 0 | - | - | |
F07 | 25 | 5 | 3 + 4 + 7 | 3 + 11 | |
F08 | 7 | 0 | Nil | Nil | |
F09 | 40 | 9 | - | - | |
F10 | 68 | 8 | 3 + 4 + 7 | 3 + 11 | |
Combined Fields | 34 | 6 | |||
1 FRAC code 3 = demethylation inhibitor, 4 = phenylamide, 7 = succinate dehydrogenase inhibitor, 11 = quinone outside inhibitor.
2 A dash (-) indicates information has not been provided.
Conclusion
This is the first report of decreased fungicide sensitivity of barley net blotch in Queensland. It reflects reports from Western Australia and South Australia and indicates a need to monitor disease across states to understand potential links to the emergence of decreased sensitivity and the risks to disease control.
The detection of net blotch with decreased sensitivity to Group 3 and Group 7 fungicides across southern Queensland emphasises the need to reduce reliance on fungicides for disease control by growing less susceptible varieties and employing integrated disease management strategies. When fungicides are necessary, their management should include rotating modes of action and using mixtures when available. These strategies need to be considered across seasons. Detailed management guidelines are available through the Australian Fungicide Resistance Extension Network (AFREN) (Ireland et al., 2021).
The greater frequency of decreased sensitivity to Group 3 fungicides compared to Group 7 fungicides likely reflects the usage of each across seasons. However, the detection of decreased sensitivity to Group 7 fungicides was not expected based on limited Group 7 use.
The extensive assessment of decreased sensitivity frequencies provides a baseline for net blotch in the southern Queensland region, with further monitoring required to understand the impacts of fungicide applications and variation across seasons.
Growers concerned about fungicide resistance are encouraged to submit samples through AFREN, or to contact their regional experts.
References
Ireland KB, Beard C, Cameron J, Chang S, Davidson J, Dodhia KN, Garrard TA, Hills AL, Hollaway G, Jayasena KW, Kiss L, Mair W, Marcroft SJ, M, MS, Milgate A, Poole N, Simpfendorfer S, Snyman L, Thomas GJ, Wallwork H, Van de Wouw AP, Zulak KG and Lopez-Ruiz FJ, 2021. Fungicide resistance management in Australian grain crops. Grains Research and Development Corporation, Australia.
Knight N, Adhikari K, Mair W, Lopez-Ruiz F and Taylor D (2022) Field profiling for net blotch fungicide resistance in the low-medium rainfall zone. GRDC Grains Research Update, Online.
Knight N, Mair W, Chandra K and Lopez-Ruiz F (2021) New horizons in the detection of fungicide resistance – combining genetic testing with in vitro assessment of fungicide performance. GRDC Grains Research Update, Crown Perth, Burswood.
Knight NL, Adhikari KC, Dodhia KN, Mair WJ and Lopez-Ruiz FJ (2024) Workflows for detecting fungicide resistance in net form and spot form net blotch pathogens. Pest Manag. Sci. 80:2131-2140.
Mair WJ, Deng W, Mullins JGL, West S, Wang P, Besharat N, Ellwood SR, Oliver RP and Lopez-Ruiz FJ (2016) Demethylase inhibitor fungicide resistance in Pyrenophora teres f. sp. teres associated with target site modification and inducible overexpression of Cyp51. Front. Microbiol. 7:1279.
Mair WJ, Thomas GJ, Dodhia K, Hills AL, Jayasena KW, Ellwood SR, Oliver RP and Lopez-Ruiz FJ (2020) Parallel evolution of multiple mechanisms for demethylase inhibitor fungicide resistance in the barley pathogen Pyrenophora teres f. sp. maculata. Fungal Genet. Biol. 145:103475.
Mair WJ, Wallwork H, Garrard TA, Haywood J, Sharma N, Dodhia KN, Oliver RP and Lopez-Ruiz FJ (2023) Emergence of resistance to succinate dehydrogenase inhibitor fungicides in Pyrenophora teres f. teres and P. teres f. maculata in Australia. bioRxiv.
Acknowledgments
The research undertaken as part of this project is made possible by the significant contributions of growers and advisors through both field identification and access, the authors would like to thank them for their support. The project was funded by the University of Southern Queensland Capacity Building Grant (2022). The authors would like to specifically thank Matthew Skerman (Nutrien) and Russell Wood (Wood Ag) for field identification, and Wesley Mair, Kejal Dodhia and Lincoln Harper (CCDM, Curtin University) for excellent technical support.
Contact details
Noel Knight
Centre for Crop Health
University of Southern Queensland
Email: noel.knight@unisq.edu.au
Date published
July 2024