Farming systems can affect soil biology, soil pathogens and system resilience
Farming systems can affect soil biology, soil pathogens and system resilience
Author: Gupta V.V.S.R. (CSIRO), Qi Yang (CSIRO), Ruth Gomez Exposito (CSIRO), Stasia Kroker (CSIRO), Marcus Hicks (CSIRO), Bhanu Nidumolu (CSIRO), Mark Farrell (CSIRO), John Kirkegaard (CSIRO), Alan Richardson (CSIRO), Lindsay Bell (CSIRO) | Date: 11 Feb 2025
Take home messages
- Increasing the diversity in cropping systems enhanced soil (micro)biological diversity and resilience
- More carbon (C) inputs through extended plant presence promotes greater activity of soil microbes
- Long fallows and systems with multiple no-crop periods decreased biological functions, diversity and resilience
- Nitrogen (N) fertilizer management influenced microbial activity and resilience
- Our goal is to develop effective indicators to assess the biological health of cropping systems.
Background
Sustaining and enhancing soil biological function is integral for the productivity and sustainability of agricultural systems, offering opportunities to build soil carbon and enhance overall ecosystem health. Soil microbial communities underpin many essential soil processes to support and maintain productive grain production systems including (i) the cycling of carbon and nutrients, (ii) suppression of diseases or pathogens and (iii) tolerance against abiotic stresses. These soil processes are vital for the sustainability of Australian agriculture and its interaction with overall ecosystem health on a landscape scale. Soil microbial communities (microbiomes) are multifunctional, performing a range of processes and activities, and include vast genetic, metabolic and physiological diversity. While in many cases groups of microbes perform similar functions, having greater diversity and some redundancy among the species present, and the physical and chemical characteristics of the soil influences the resilience of the soil’s biological functions and the cropping system. The capacity of a soil to withstand (resistance) and recover (resilience) from external stresses, including environmental, physical and chemical stresses, and maintain its biological functions can affect system productivity. Predicted climate scenarios, including changes to rainfall distribution and levels, and growing season temperatures are expected to amplify these qualities.
Both human induced (through management practices) and environmental stimuli and stress events can alter the composition, diversity and activity of soil microorganisms. In agricultural soils, the depletion of carbon rich microsites and changes to quality of C inputs can affect the distribution, diversity and metabolic status of microbial communities and resilience. Recent research suggests that increasing the crop diversity and/or increasing duration (reducing fallow periods), will bring improvements in the soil microbiome (bacteria, fungi, archaea and protist) diversity and their beneficial functions such as nutrient supply, plant growth and disease suppression. Conversely, systems with little crop diversity and/or with long fallow periods and less C inputs are likely to deplete the soils beneficial biological functions. Improving both resistance and resilience of soil systems to stresses can inform management decisions to better harness soil biology and its functional capacity in agricultural systems. A functional microbial ecology approach which integrates responses in the diversity, abundance and activity of key functional groups of microbiota can provide a useful measure of soil health and its resilience (Figure 1).
The main objective and first step in this work was to assess how the diversity and crop intensity of different farming systems affect the soil biology and its functions with a focus on nutrient supply, microbial C turnover (links to SOC sequestration) and resilience against environmental stresses. This project utilized treatments in existing long-term farming systems experiments in ongoing GRDC investments (Projects: DAQ2007-004RMX, 9175150/CFF00011) in the northern grains region.
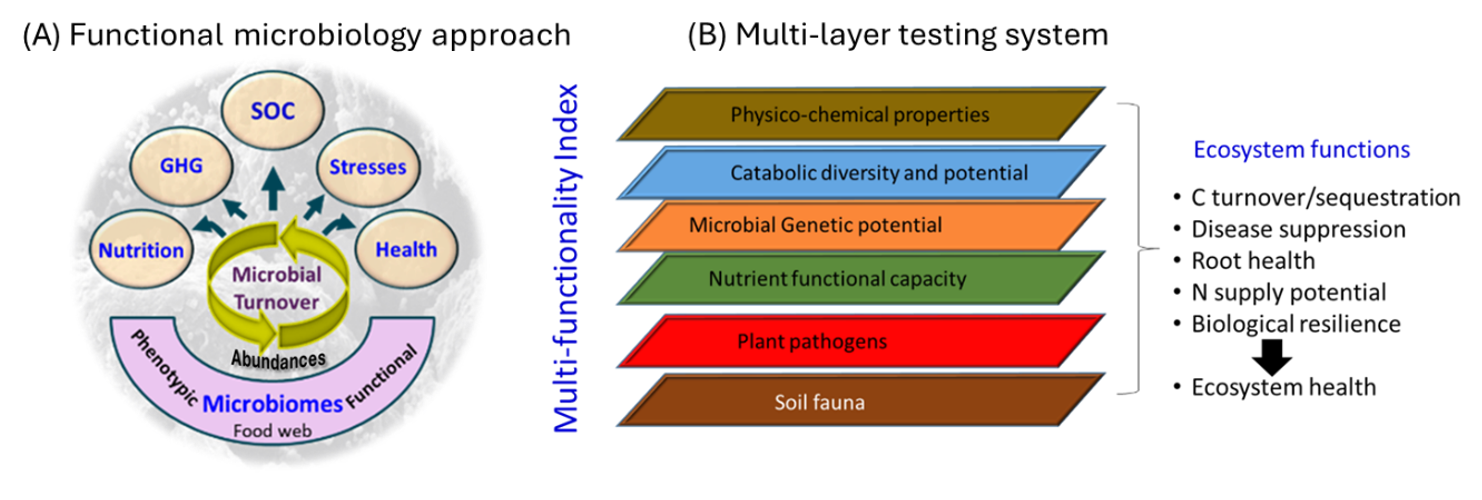
Figure 1. A pictorial representation of (A) the functional microbial ecology approach which helps integrate responses in the diversity, abundance and activity of different functional groups of microbiota, and (B) multilayer testing system to quantify responses in soil biodiversity, genetic potential for various beneficial and pathogenic functional groups, biotic communities and biological functions.
SOC – Soil organic C sequestration, GHG – Green House Gas emissions.
What did we do?
During November 2023 and April 2024, surface 0–10cm soil samples were collected from selected treatments in long-term farming system experiments conducted at Pampas, Qld and Iandra (Greenethorpe), NSW. A total of 7 to 9 cropping systems varying in crop diversity, intensity, nutrient (N) inputs and grazing were sampled. These sampling times allowed us to investigate how 6–8 years of prior management of these farming systems influenced the biological functional capacity, but particularly the crop’s ability to access the necessary nutrients and recruit beneficial microbial groups in the rhizosphere to help with plant nutrition and health.
Table 1. Details of cropping system treatments analysed for soil biological properties from the farming system field experiments at Pampas in Queensland and Iandra in New South Wales.
Treatment | Started | Sampled | Total number of crops | Number of plant types | Plant types | Fertiliser | Grazing |
|---|---|---|---|---|---|---|---|
Location: Pampas, Qld | |||||||
Conventional | 2015 | 2023 | 7 | 2 | wheat, chickpea | 50th percentile yield potential | Ungrazed |
High Intensity (HI) | 2015 | 2023 | 12 | 5 | wheat, mungbean, sorghum, chickpea, barley | Ungrazed | |
High Intensity+High Diversity | 2015 | 2023 | 12 | 8 | fieldpea, mungbean, sorghum, chickpea, sunflower, millet, wheat, fababean | Ungrazed | |
High Diversity (HD) | 2015 | 2023 | 8 | 8 | fababean, cotton, mungbean, soybean, sorghum, chickpea, wheat | Ungrazed | |
Low Intensity (LI) | 2015 | 2023 | 6 | 5 | maize, mungbean, cotton, millet, wheat | Ungrazed | |
Permanent Fallow (PF) | 2015 | 2023 | 0 | 0 | No crop | Nil | Ungrazed |
Pasture (PP) | 2015 | 2023 | 2 | 2 | Rhodes grass, bambasti, Burgundy bean and snail medic | Nil | Ungrazed |
Location: Iandra, NSW | |||||||
Continuous Wheat | 2018 | 2024 | 6 | 1 | wheat | N2 | Ungrazed |
Baseline N2 (Base_N2) | 2018 | 2024 | 6 | 2 | canola, wheat, wheat | N2 | Ungrazed |
Baseline N7 (Base_N7) | 2018 | 2024 | 6 | 2 | canola, wheat, wheat | N7 | Ungrazed |
Intesive Baseline N2 (IntBase_GR_N2) | 2018 | 2024 | 6 | 2 | canola, wheat, canola, wheat | N2 | Grazed |
Intesive Baseline N7 (IntBase_GR_72) | 2018 | 2024 | 6 | 2 | canola, wheat, canola, wheat | N7 | Grazed |
Intesive Baseline N2 (IntBase_NG_N2) | 2018 | 2024 | 6 | 2 | canola, wheat, canola, wheat | N2 | Ungrazed |
Intesive Baseline N7 (IntBase_NG_N7) | 2018 | 2024 | 6 | 2 | canola, wheat, canola, wheat | N7 | Ungrazed |
Diverse Low value legume (DivLV) | 2018 | 2024 | 6 | 3 | fababean, canola, wheat | N2 | Ungrazed |
Diverse High value legume (DivHV2) | 2018 | 2024 | 6 | 2 | chickpea, wheat | N2 | Ungrazed |
Note: N2 and N7 denote N-strategies targeting a decile 2 yield potential or decile 7 yield potential. Grazing in canola is finished and animals removed before GS51 (flower buds visible from above) and in wheat before GS31. | |||||||
Existing single measurement of soil health indicators are inadequate to determine the capacity of a soil to deliver key functions while the extensive information generated by microbial DNA sequencing approaches are often too complex to derive simple practical messages. Using a multi-layer testing system involving measures for functional capacity and genetic potential of the microbial communities, linked to physical and chemical characteristics, soil samples were analysed to derive integrated measures of biological functional capacity, diversity and resilience. For example, microbial biomass C and catabolic diversity and potential (i.e. capacity to breakdown organic materials into energy) were measured to quantify changes in microbial C and N turnover as potential indicators of N supply capacity and C sequestration potential. We used functional gene-qPCR methods to quantify the abundance of specific microbial genes involved in N mineralization and fixation, P release, and S oxidation as measures of microbial potential to drive nutrient supply processes. The soil bacterial (16S rRNA gene) and fungal (ITS region) community composition was determined using group specific amplicon sequencing techniques. We also used DNA from soil samples collected during previous seasons from the Pampas experiment (2015, 2018 and 2021) to explore changes in the composition and diversity of soil bacterial community.
What did we find?
Microbial C turnover – microbial biomass and catabolic diversity
Microbial biomass carbon (MB-C) and nitrogen (MB-N) levels showed clear and significant differences between farming systems at both sites (Table 2). At Pampas, MB levels were highest in the Perennial Pasture (PP) (400 µg C/g soil) and lower (by 64%) under the Permanent Fallow (PF) treatment (144 µg C/g soil). Soils from various cropping systems generally had lower MB levels, compared to perennial pasture (by an average 46%), with the lowest MB levels observed under the Low Intensity (LI) system (147 µg C/g soil). At the Iandra experimental site, MB levels were significantly highest (463 µg C/g soil) in soils from the grazed and early sown system with conservative N supply (i.e., IntBase_GR_N2). Across all systems those with a higher N fertilizer approach (N7 – targeting decile 7 years) were generally lower compared to that in the N2 systems (i.e. targeting decile 2 years). Higher rates of N fertilizer may have altered the assimilation vs. respiration processes in microbes resulting in lower MB levels. Intensive Base systems generally supported higher MB levels at both N-management strategies; 60% and 37% higher compared to the respective Baseline systems.
The measures of microbial catabolic potential (AMR) and the diversity of soil microbial community to utilize multiple C substrates (CMD) and C mineralisation potential (e.g., Substrate Induced Respiration (SIR), Potential Min C) reflect the capacity of the microbes to turnover or metabolise carbon. These all showed significant variation between farming systems at both sites (Table 2). In the Pampas experiment, they were reduced in the Low Intensity system even when compared to the Conventional Cropping system which suggests that the microbial community is highly C limited. The biologically available C pools may be declining faster than the C inputs from the crops, which is further confirmed by the higher CMD and AMR values for the Higher Intensity systems. Results for the Higher Diversity and HI+HD (High Intensity + High Diversity) systems suggest that increasing the diversity of crops alone may not be sufficient to maintain microbial C turnover.
At the Iandra site, crop intensification combined with grazing (i.e., IntBase_GR) significantly increased both the AMR and CMD, whereas the higher N fertilizer treatment showed lower catabolic properties measures. Soil habitat characteristics such as pH and Cation Exchange Capacity (CEC) have been shown to influence the catabolic diversity indices (Sradnick et al., 2013). Thus, the observed differences in catabolic diversity from the community level physiological profiles across different farming system treatments and two soil types could be partly attributed to variations in the genetic composition of microorganisms (Gupta et al., 2010; Griffiths and Philippot, 2013), as well as the quantity and quality of C inputs and the habitat characteristics. Farming systems with higher catabolic diversity such as IntBase_N2 and DivLV also showed higher resilience in microbial activity, whereas N7 systems generally showed lower resilience (data not shown).
Table 2. Results for soil chemical and biological properties as influenced by different cropping systems in field experiments at Pampas in Queensland and Iandra in New South Wales.
Treatments | Organic C | Total N | MB-C | MB-N | AMR | SIR | CMD | Min N | Potential Min C |
|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (µg C/g) | (µg N/g) | µg CO2/g/day |
| (µg N/g) | (µg CO2-C/g/day) | |
Location: Pampas, Qld | |||||||||
Conventional | 1.561 | 0.110 | 223.5 | 31.9 | 4.46 | 10.72 | 11.67 | 42.6 | 2.39 |
HI | 1.628 | 0.115 | 241.7 | 34.5 | 4.57 | 11.52 | 11.58 | 15.2 | 2.58 |
HI+HD | 1.598 | 0.113 | 233.4 | 33.3 | 3.88 | 11.42 | 9.92 | 9.2 | 2.58 |
HD | 1.598 | 0.125 | 230.1 | 32.9 | 3.30 | 8.97 | 8.08 | 44.2 | 3.55 |
LI | 1.498 | 0.097 | 146.9 | 21.0 | 2.24 | 4.18 | 4.50 | 13.8 | 2.36 |
PF | 1.305 | 0.083 | 144.2 | 20.6 | 0.98 | 1.82 | 1.33 | 13.5 | 1.28 |
Pasture | 1.855 | 0.125 | 400.2 | 57.2 | 3.50 | 7.88 | 9.33 | 4.2 | 3.97 |
F-test | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 |
LSD (P<0.05) | 0.087 | 0.009 | 46.3 | 6.6 | 0.03 | 2.853 | 1.97 | 8.78 | 1.14 |
Location: Iandra, NSW | |||||||||
Continous Wheat | 1.343 | 0.106 | 341.0 | 48.7 | 5.37 | 13.26 | 16.89 | 7.8 | 13.5 |
Base_N2 | 1.320 | 0.104 | 287.0 | 41.0 | 5.57 | 10.22 | 16.00 | 7.0 | 8.7 |
Base_N7 | 1.270 | 0.104 | 236.6 | 33.8 | 4.31 | 9.54 | 11.89 | 9.5 | 7.1 |
IntBase_GR_N2 | 1.520 | 0.123 | 463.2 | 66.2 | 6.52 | 15.91 | 20.00 | 6.9 | 16.7 |
IntBase_GR_N7 | 1.537 | 0.127 | 358.0 | 51.1 | 7.25 | 15.06 | 18.78 | 13.4 | 11.4 |
IntBase_NG_N2 | 1.290 | 0.100 | 324.0 | 46.3 | 4.49 | 11.34 | 12.56 | 14.5 | 13.6 |
IntBase_NG_N7 | 1.257 | 0.099 | 239.0 | 34.1 | 3.58 | 5.62 | 9.56 | 32.4 | 9.0 |
DivLV | 1.330 | 0.103 | 332.0 | 47.4 | 4.82 | 11.96 | 14.11 | 8.6 | 12.1 |
DivHV2 | 1.290 | 0.105 | 316.0 | 45.1 | 5.35 | 13.29 | 15.33 | 7.5 | 12.8 |
F-test | 0.006 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.08 |
LSD (P<0.05) | 0.194 | 0.014 | 73.7 | 10.5 | 0.909 | 2.81 | 3.19 | 6.8 | |
Notes: MB – Microbial Biomass, AMR – Average Metabolic Response, SIR – Substrate Induced Respiration,
CMD – Community Metabolic Diversity, Min N – mineral N, Potential Min C – potentially mineralizable carbon.
The responses in soil organic C and total N levels to different farming system treatments generally reflected trends seen in the MB-carbon levels. For instance, at the Pampas site, organic C levels in soils under various cropping systems and the Permanent Fallow system were 15% and 30% lower than in the Perennial pasture system, respectively, compared to those in the PP system. In the Iandra experiment, Intensive-Base system soils showed higher organic C and total N levels compared to other farming systems (Table 2). The microbial quotient (MQ) is one of the important derived measures used to indicate changes in MB-carbon, the potential for microbial carbon turnover, and overall soil quality or health in different farming systems. Unlike the absolute values of MB, MQ being a ratio, avoids the issues associated with comparing values across soils and systems with varying different total soil organic C levels. In general, MQ values were lower for soils in the Pampas experiment (average 1.44%±0.14) compared with values observed for Iandra soils (2.37%±0.15). The lowest MQ value was observed in Permanent Fallow soils at Pampas (0.98%) and the IntBase_N7 treatment at Iandra site (1.86%). Lower MQ and declining catabolic properties generally indicate that soil organic matter is being used in an exploitative manner and microbial pools are declining faster than changes in the total organic C and suggests the necessity to implement management practices that would increase MB levels and associated benefits.
Microbial diversity and composition of soil bacterial and fungal communities
The diversity of soil microbial community has been linked to biological functions such as disease suppression, C sequestration and functional resilience to crop management practices (Gupta et al., 2019; Rieke et al., 2022). This research has shown that farming systems can significantly influence the soil bacterial and fungal communities (Figure 2). For example, at the Pampas site, High Intensity and High Diversity systems showed significantly higher bacterial and fungal diversity (Figure 2A & 2B). Both bacterial and fungal diversity were lowest in the Permanent Fallow system, and the diversity in the Perennial Pasture system was similar to that in the Low Intensity and Conventional Cropping systems. Increasing crop rotational diversity increases quantity, quality and diversity of crop residues, which supports more diverse microbial communities. Additionally, crop type-based variation in microbial diversity contributes to higher diversity in High Diversity systems. In contrast, the higher microbial diversity in High Intensity systems can be attributed to longer periods of crop presence.
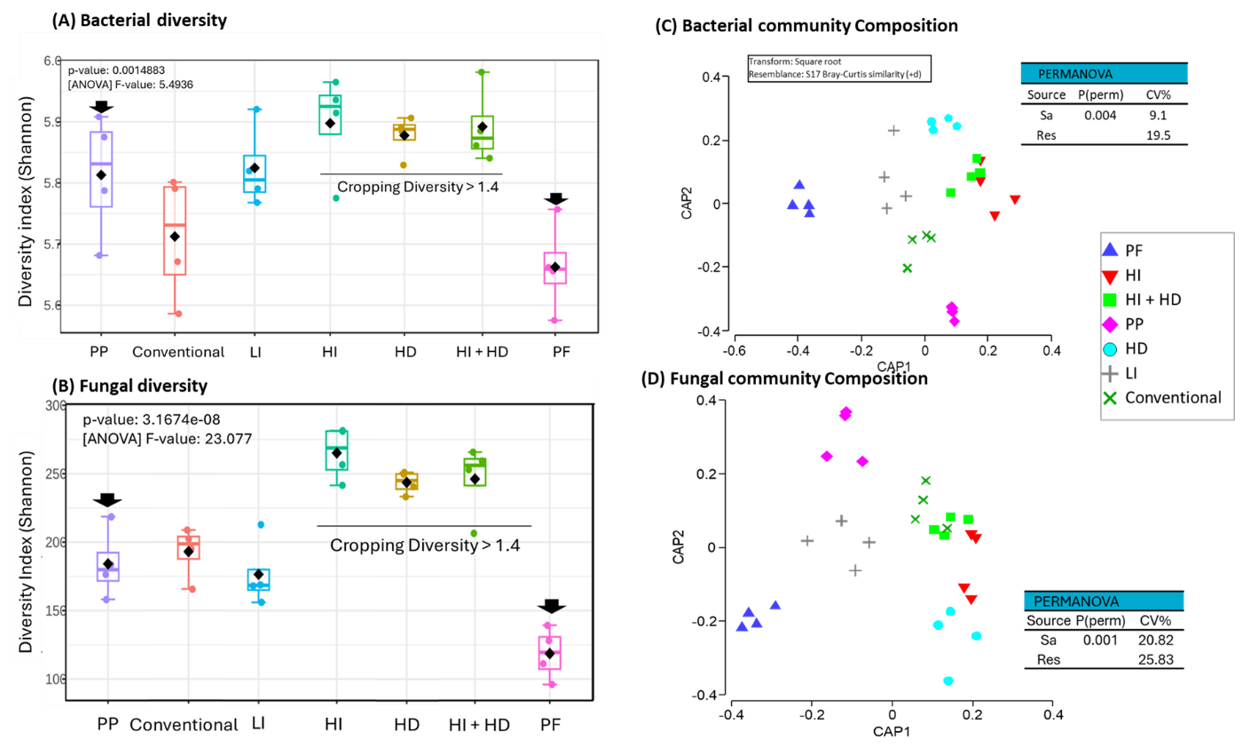
Figure 2. Effect of 8 seasons of cropping intensity and diversity treatments on soil for (A) bacterial (16s rRNA) and (B) fungal (ITS region) community diversity, and for (C) bacterial community composition and (D) fungal community composition in surface soils collected during November 2023 from selected treatments in the field experiment at Pampas, Qld.
Soils from different farming systems also showed significant differences in the composition of bacterial and fungal communities (Figure 2C & 2D). Bacterial community composition in the Low Intensity and Conventional Cropping systems were similar, and they were different to that in soils under the Permanent Fallow system. Both High Intensity and High Intensity + High Diversity systems showed similar community composition, but they were different to that in the High Diversity system soils. Results for the previous season’s samples showed significant differences in bacterial community composition after 3 and 6 years of the farming system treatments. Research in the rainfed cropping systems in southern Australia indicated that the development of agronomically effective biological disease suppression capability would take 5–7 years/seasons (Gupta et al., 2019). In this study, changes in the bacterial diversity to different farming systems appeared to plateau after 6 years of experiment (data not shown).
Changes in the populations of soilborne fungal pathogens with different farming systems treatments generally varied depending upon the previous crop type (data not shown). In contrast, a significant decline in the populations of nematodes (Pratylenchus thornei) was observed in the Permanent Pasture system at the Pampas site.
Microbial genetic potential and functional capacity for nutrient cycling and uptake
Farming system history also influenced the abundances of functional genes responsible for different biological processes involved in N, P and S cycling (data not shown). Soils from the Pampas experiment showed lower abundances of all the measured genes (11 genes involved in N, P and S cycling) in the Permanent Fallow and Low Intensity treatments and highest abundance in the High Intensity system. Whereas High Intensity and High Diversity treatments harboured higher abundances of functional genes involved in nitrification, organic N mineralization, P release and sulfur oxidation. Functional genes relevant for denitrification were higher in the Perennial Pasture and High Intensity systems compared to Low Intensity and Conventional Cropping systems. The impact of farming system on activities of different enzymes involved in C cycling, N, P and S mineralization differed between farming systems and the two sites (Pampas and Iandra). Generally, enzyme activities were lower in the Permanent Fallow and Low Intensity in the Pampas experiment and in the IntBase_N2 treatment in the Iandra experiment (data not shown).
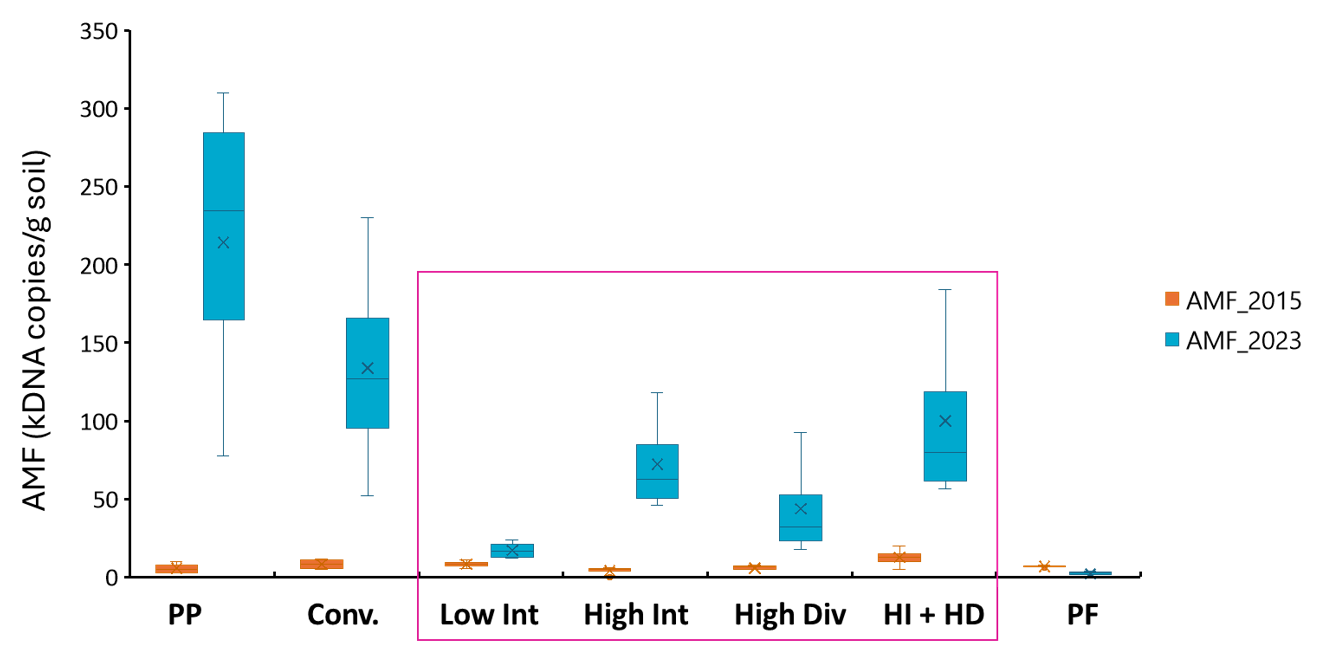
Figure 3. Abundance of arbuscular mycorrhizal fungi (AMF) in surface soils as influenced by different cropping intensity and diversity treatments in the samples collected at the start of the experiment (2015) and in November 2023 from the field experiment at Pampas, Qld.
The symbiotic relationship between plants and arbuscular mycorrhizal fungi (AMF) helps plants with efficient acquisition of nutrients such as phosphorus and zinc, and to also overcome abiotic stresses. After 8 years of the experiment, the abundance of AMF community showed significant responses to different farming systems (Figure 3). For example, the Perennial Pasture system showed highest abundance whereas the Low Intensity system showed significantly lower abundance. There was a slight decline in the AMF abundance in 2023 compared to the start of the experiment for the Permanent Fallow system. Long periods of plant-free fallows and non-host crops have been shown to reduce viable AMF propagules and poor colonization of following crops (Thompson 1987). Research during the last two decades suggests that the magnitude of benefits from AMF may vary in the different cropping regions of Australia.
Microbial community structure
Microbial community network analysis provides insights into how different members and groups of microbe’s interact with each other, with their environment and the derived consequences to biological functions, in particular the resilience of the communities and functions. Results for the soil bacterial community networks showed distinct relationship between some of the key network properties such as nodes (i.e. number of significant microbes) and number of connections between nodes with the Intensity and Diversity properties of the different farming systems (Figure 4). The Permanent Fallow, Conventional Cropping and Low Intensity farming systems with lower crop intensity and diversity showed lower bacterial network metrics, whereas High Intensity and High Diversity systems showed higher network metrics indicating a well-connected bacterial community. It is recognized that microbial communities with larger number of nodes and greater connectivity are generally more robust or resilient to short-term changes because in well-structured communities all microbes are able to use resources efficiently, perform functions, resist pathogen invasions and tolerate higher levels of stress. Previous research has shown that disease suppressive soils generally support a well-connected and stable bacterial and fungal community structure thereby reducing the impacts of soilborne diseases even in the presence of pathogen inoculum (Penton et al., 2014; Gupta 2014). Despite the presence of plants throughout the experimental period, lower bacterial diversity metrics in the Perennial Pasture system are partly attributed to the lack of annual disturbance compared to that in the cropping systems.
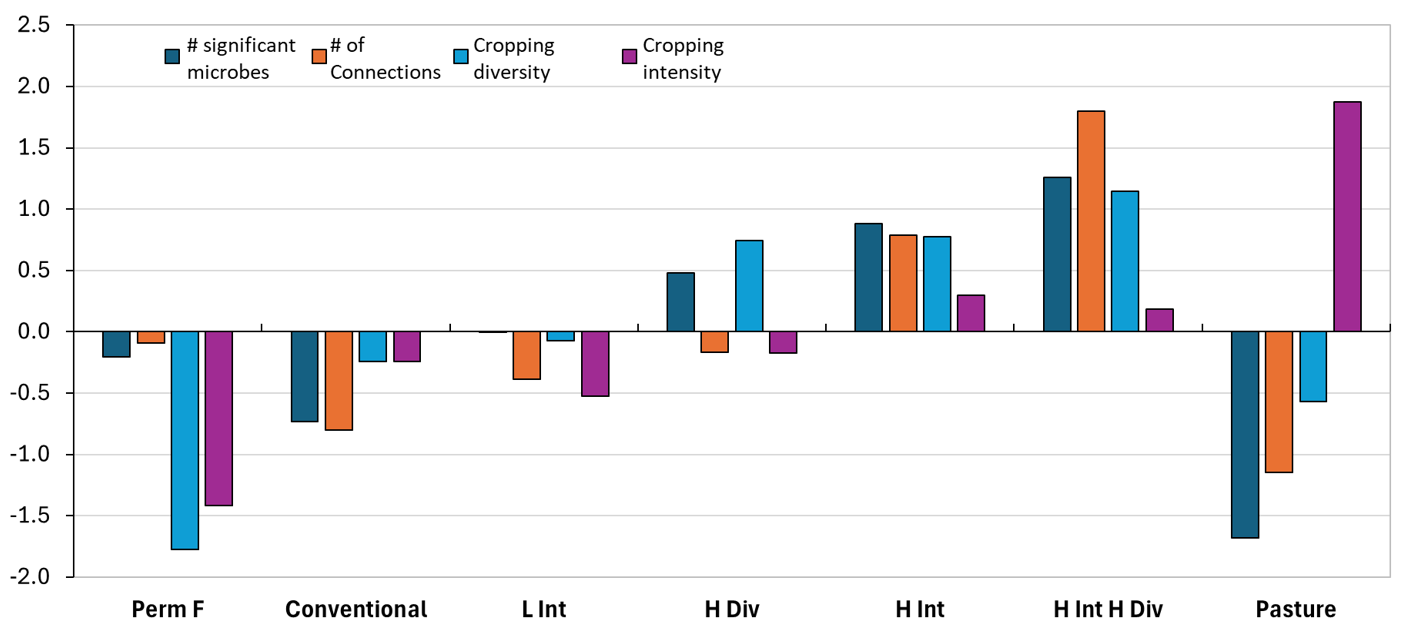
Figure 4. Effect of 8 seasons of cropping intensity and diversity treatments on soil bacterial and fungal community network complexity in surface soils collected during November 2023 from selected treatments in the field experiment at Pampas, Qld. Nodes (number of significant microbes) and Edges (number of connections) represent microbial community network properties, whereas diversity and intensity metrics indicate about the nature of the cropping system. Data for individual functions standardised to a common scale by taking their mean across all the soils tested in this study using a z-score transformation (Bradford et al., 2014).
Conclusions
Soil microbial communities are the foundation of soil food webs through the intricate interactions among microbial groups, both beneficial and deleterious, and soil fauna, and they regulate essential soil biogeochemical cycles essential for plant nutrition, health and maintaining productive grain production systems. Key findings include:
- A clear and significant influence of the management interventions, such as increasing crop diversity and intensity, on biological diversity, functions and resilience was observed.
- While more C inputs through longer-season crops/pastures increased microbial activity and microbial diversity, fallows decreased biological functions, diversity and resilience.
- In general, increasing crop diversity in a farming system enhanced soil microbial diversity and resilience, and systems with a higher N fertilizer approach (N7) were generally lower in microbial biomass, activity and resilience.
While the information on overall microbial diversity would provide in-depth knowledge about microbial communities, choosing microbial tests that match key functions of interest for soil health parameters will be more useful to develop specific microbial indices and to develop recommendations to improve biological functions. Additionally, as microbial community data generally shows strong geographic and soil-based differences, it is important that such data is interpreted in a functionally-specific (e.g., denitrification, N mineralization) and context-specific (e.g., crop rotation, tillage, chemical effects) manner. For example, crop intensification, diversity and N management showed significant changes in microbial C turnover, microbial community diversity and composition. By linking specific microorganisms that were enriched in High Intensity and High Diversity systems with their functional capabilities (such as the ability to produce extracellular polymeric substances) that promote C accumulation, provides context to evaluate management practices for SOC sequestration. Similarly, information on nutrient supply capacity and microbial community structure can provide the status of soil functional capacity and resilience in a farming system. The multilayer functional microbial ecology approach used in this can provides integrated measures of biological health and resilience of cropping systems.
Future directions
The initial findings from the first step of this work on the impact of farming systems on key biological functions and resilience need now to be integrated across different layers. This will help develop useful metrics that can be directly linked to the farming systems, enhancing their evaluation and management through informed decision making.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of collaborators of the parent projects (DAQ2007-004RMX and 9175150/CFF00011) and the support of the GRDC, the author would like to thank them for their continued support. The research conducted by Qi Yang was supported through the CSIRO-CERC fellowship. We acknowledge the various collaborators for their help with collecting soil samples and providing field experimental data, and farmer collaborators for hosting the farming systems experiments across the regions. Specific acknowledgments to Anna Williams and David Coleman with the experiment at Pampas, Qld and Gabe Brown and Li Xiaoxi with the field experiment at Iandra, NSW.
References
Bradford MA, Wood SA, Bardgett RD and Jones TH (2014) Discontinuity in the responses of ecosystem processes and multifunctionality to altered soil community composition. Proceedings of the National Academy of Sciences, 111, 14478–14483.
Griffiths BS and Philippot L (2013) Insights into the resistance and resilience of the soil microbial community. FEMS Microbiology Reviews. 37: 112–129.
Gupta V, Roper M, Thompson J (2019) Harnessing the benefits of soil biology in conservation agriculture. In ‘Australian Agriculture in 2020: From Conservation to Automation’. (Eds J. Pratley, J. Kirkegaard) pp. 237–253. (Agronomy Australia and Charles Sturt University: Wagga Wagga, NSW).
Gupta VVSR, Penton CR, Lardner R and Tiedje J (2010) Catabolic and genetic diversity of microbial communities in Australian soils are influenced by soil type and stubble management. Proceedings of the 19th World Congress of Soil Science; Soil Solutions for a Changing World; ISBN 978-0-646-53783-2.
Gupta VVSR (2014) A molecular approach to unravel the dynamics of disease suppressive microbial communities. Final report for the GRDC project CSP00135.
Gupta VVSR, Nidumolu B, Kroker S, Hicks M and Llewellyn R (2024) Regenerative opportunities for building soil biological resilience – a case study in the low-rainfall zone in southern Australia. GRDC update on 6th February, Adelaide, SA.
Penton CR, Gupta VVSR, Tiedje JM, Neate SM, Ophel-Keller K, Gillings M, Harvey P, Pham A and Roget DK (2014) Fungal community structure in disease suppressive soils assessed by 28S LSU gene sequencing. PLoS ONE 9(4): e93893.
Rieke EL, Rieke EL, Cappellazzi SB, Cope M, Liptzin D, Mac Bean G, Greub KLH, Norris CE, Tracy PW, Aberle E (2022) Linking soil microbial community structure to potential carbon mineralization: A continental scale assessment of reduced tillage. Soil Biology and Biochemistry. 168. 108618.
Sprunger CD and Martin TK (2023) An integrated approach to assessing soil biological health. Advances in Agronomy. 182: 131–168.
Sradnick A, Murugan R, Oltmanns M, Raupp J and Joergensen RG (2013) Changes in functional diversity of the soil microbial community in a heterogeneous sandy soil after long-term fertilization with cattle manure and mineral fertilizer. Applied Soil Ecology. 63:23–28.
Thompson JP (1987) Decline of vesicular-arbuscular mycorrhizae in long fallow disorder of field crops and its expression in phosphorus deficiency of sunflower. Australian Journal of Agricultural Research. 38: 847–867.
Contact details
Gupta Vadakattu
CSIRO Agriculture & Food
Waite campus
Urrbrae SA
gupta.vadakattu@csiro.au
Date published
February 2025
GRDC Project Code: CSP2401-015RTX, DAQ2007-004RMX, CSP1703-007RTX,
