Nitrogen volatilisation: Factors affecting how much N is lost and how much is left over time
Author: Graeme Schwenke, NSW DPI | Date: 25 Jul 2014
Take home messages
Fertiliser nitrogen may be lost from the soil in several different ways, including; ammonia volatilisation, nitrate leaching and nitrate denitrification. Factors affecting these losses include fertiliser compound, fertiliser form, type of application, timing of application, soil properties, rainfall amount and intensity, and temperature and wind after application.
Field trials on northwest NSW cracking clay soils (Vertosols) during 2011-2013 showed that surface application of urea led to ammonia volatilisation averaging 11% N loss when applied to fallow soils, and 5% N loss when applied to tillering wheat crops. Compared to urea, losses from ammonium sulfate were less, except when the soil contained >2% calcium carbonate (lime). Nitrogen losses from ammonium sulfate applied to fallow soils averaged >20% where soils contained >10% calcium carbonate. Calcium carbonate content did not affect losses from urea or other nitrogen fertilisers trialled.
A combined statistical analysis of all plots that had <2% calcium carbonate showed that ammonia volatilisation loss was principally affected by; (a) the presence of a crop, (b) fertiliser type, and (c) the average windspeed at ground level. Losses were greater in fallow paddocks than in-crop, and greater under windy conditions. A separate analysis of all urea plots in the study found that N loss was mainly influenced by, (a) the presence of a crop, (b) soil texture (sandier = greater loss), and (c) soil moisture content at spreading (wetter soil = greater loss).
After the month of volatilisation measurements, most of the non-volatilised applied nitrogen was recovered in the topsoil or plant tissue. The exception was where paddocks had had intense rainfall which likely caused nitrate leaching and denitrification.
Ammonia volatilisation: a chemical process that occurs at the soil surface when ammonium from urea or ammonium-containing fertilisers (e.g. urea) is converted to ammonia gas at high pH. Losses are minimal when fertiliser is incorporated, but can be high when fertiliser is surface-applied.
Nitrate denitrification: a biological process that occurs within the soil profile wherever there is sufficient available nitrate, labile carbon substrate, and low oxygen conditions such as in slowly draining soils. Losses are minimal in most dryland cropping soils, but may be high in waterlogged conditions.
Nitrate leaching: a physical process that occurs with the drainage of water through the profile. While nitrate movement within the profile is common in cracking clay soils, large-scale loss of nitrate below the root zone is minimal in most conditions.
Ammonia volatilisation research
Dryland grain growers on cracking clay soils (Vertosols) in the northern grains region of Australia are increasingly using pre-crop broadcasting and in-crop topdressing of nitrogen (N) fertilisers. Surface-application risks gaseous loss of the applied N via ammonia volatilisation, but the magnitude of N loss is unknown. Both soil properties and environmental conditions are known to influence ammonia volatilisation, so measurements need to be field-based and non-intrusive. In 19 separate field experiments, we used a micrometeorological technique to measure cumulative ammonia loss over a month after application of 2–6 fertiliser products in 10 fallow paddocks, 7 mid-tillering wheat crops, and 2 perennial grass-based pastures.
Cumulative ammonia volatilisation results for all fertiliser treatments in all field experiments are summarised in Figure. 1. In general, the magnitude of N losses measured in this study was low compared to losses of 25% or more reported from pre-1980’s studies conducted overseas, mostly on lighter textured soil types, and not measured using micrometeorological measurement.
A soil’s affinity for ammonia/ammonium is one of the most influential factors governing the potential for ammonia volatilisation, through both cation exchange of ammonium, and physical adsorption of ammonia in very dry soils. Vertosols typically have a moderate to high soil cation exchange capacity. Critical CEC values of 25 cmol kg-1 (O'Toole et al. 1985) or 32 cmol kg-1 (Bouwman et al. 2002) have been proposed, below which ammonium adsorption by the soil is minimal, and above which ammonia loss by volatilisation is substantially reduced. We found no relationship between soil CEC and N loss in our study, probably because most soils in the study were well above these critical thresholds.
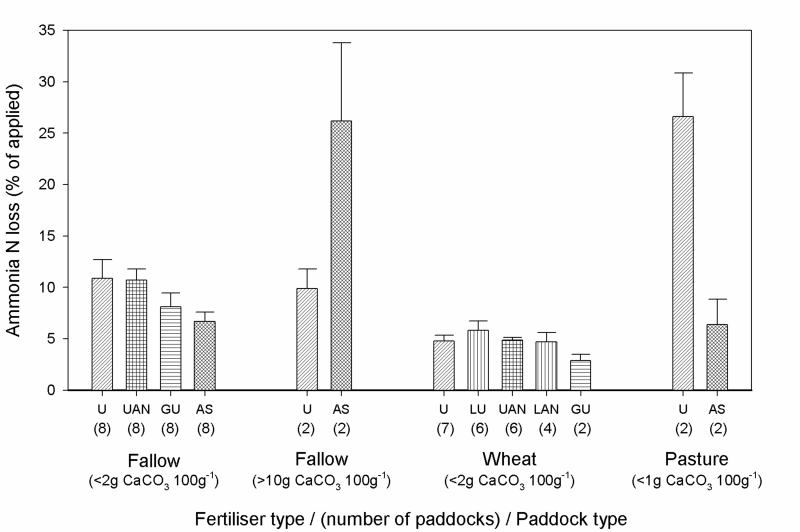
Figure 1. A summary of the cumulative N loss via volatilisation in this study, grouped by paddock type and fertiliser type in 19 separate paddock experiments (219 site-months data). Bars are means (+ standard error) of results from 2–8 paddock experiments (number of paddocks in each group is given under bar). U = urea, UAN = urea ammonium nitrate solution, GU = Green urea®, AS = ammonium sulfate, LU = urea solution, LAN = ammonium nitrate solution.
N losses from fallow soil
From our results, ammonium sulfate is the preferred N fertiliser for minimum volatilisation when used on fallowed cropping soils, provided the surface soil does not contain >2% calcium carbonate. Fallow paddocks were segregated according to surface soil calcium carbonate content, as those with >10% calcium carbonate lost considerably more ammonia from ammonium sulfate than urea (Fig. 1). There were no paddocks in the 2-10% calcium carbonate range. Calcium carbonate in soil, especially when present in clay-sized particles, enhances ammonia volatilisation from soluble ammonium sulfate because the calcium sulfate formed has a low solubility which provides a driving force to the reaction that also produces ammonium carbonate/bicarbonate and a high solution pH. Calcium carbonate levels in soil were not related to N losses from any of the other fertilisers trialled.
At five of the eight low calcium carbonate fallow paddocks, ammonia loss from ammonium sulfate was significantly less than from urea, 52% less on average. Similarly, Turner et al. (2012) measured 50% less ammonia volatilised from ammonium sulfate (2.8–12% of N applied), compared to urea at two in-crop paddocks in the Victorian Mallee. In 2013, ammonium sulfate was 36% more expensive than urea per unit N in the study region, so broadcasting it on non-calcareous fallow soils should be cost effective compared to urea. However, the benefits also need to be weighed against the extra costs of transport and spreading, since ammonium sulfate has a lower N concentration than urea. Ammonium sulfate is regarded as unsuitable for topdressing in cereal crops of the study region because it cannot be spread in as wide a swathe as urea and therefore takes longer to spread in the face of forecast rain systems.
Losses from urea averaged 11% for fallowed cropping soils (range: 5.4–19%). Coating urea with N-(n-butyl) thiophosphoric triamide (NBPT) (Green urea®) significantly reduced ammonia loss at 2 out of 8 fallow paddocks and 1 out of 3 in-crop paddocks. A chemical derivative of the NBPT has a strong urease inhibitor effect, which means that it delays the breakdown of urea into ammonium. Green urea® is mainly targeted at pasture situations where there is less option for fertiliser-soil contact without sufficient rainfall. In our study, results with Green urea® were inconsistent so may not justify the 25% higher cost at local retailers (costed in 2013). In the responsive paddocks, the reduction in N loss compared to urea averaged 61%. It is possible that average daily temperatures at the non-responsive paddocks (11–22oC) diminished the effectiveness of the urease inhibitor, as was shown for temperatures >15oC in a lab study of southern Australian soils (Suter et al. 2011). Average daily temperatures were <12oC at the responsive paddocks in our study.
Urea ammonium nitrate (UAN) should be a lower risk than urea in terms of ammonia volatilisation, because part of the compound is in the nitrate form which does not volatilise. However, ammonia losses from UAN in our study ranged from 4–15% and were similar overall to N losses from urea in both fallow and in-crop experiments (Fig. 1). Volatilisation from UAN was only significantly less than urea (31% less on average) at two out of eight fallow paddocks. Reasons for this inconsistency are unclear. Losses from UAN were 57% less than urea in two wheat paddocks studied by Turner et al. (2012) where cumulative UAN-N losses ranged from 1.8–12% of the N applied. From our results, we could not recommend UAN ahead of urea, although it is preferred by some growers due to its greater ease of application and more even distribution.
N loss from in-crop topdressing
In our study, topdressing a wheat crop had a relatively low risk of volatilisation loss, regardless of which fertiliser product was used (Fig. 1). Cumulative ammonia loss in the 7 wheat paddock experiments ranged from 2–9% of the N applied across all treatments, and averaged just 5% from urea (range: 3–8%) (Fig. 1). Total N losses may have been slightly higher in a few paddocks if measurements had continued beyond one month, as dry conditions during the month after application may have meant not all of the urea had fully hydrolysed to ammonium. The in-crop paddocks of our study tended to have lower soil moisture at fertiliser application, less rainfall after application and lower average temperatures during the measurement period than the fallow paddocks.
Only Turner et al. (2012) have previously reported field measurements of ammonia volatilisation from urea applied to a wheat crop grown on a Vertosol. In their southern Australian study, 13–23% of the urea N applied was lost from a grey self-mulching clay soil (Vertosol), with highest losses occurring when urea was applied after 21 mm of rain, and light showers followed. Volatilisation losses reported from topdressed urea on other soil types in southern Australia include 5% (Turner et al. 2012) and 10% (Turner et al. 2010) from clay loams. Bacon and Freney (1989) reported 12% N loss from urea applied at tillering to a no-till soil (soil type not given) but less than 2% from cultivated soils at tillering and from either soil when applied at ear initiation. All fallow paddocks in our study were no-till.
While we found some statistically significant differences in N loss between fertiliser products at some paddocks, the differences were not consistent, so overall, none of the alternative products could be recommended ahead of urea for use in topdressing at tillering growth stage.
N loss from pasture topdressing
The lack of rain after urea application led to high losses of ammonia from two grass-based pastures (Fig. 1). In the 2 ungrazed pasture paddocks, an average of 27% of the urea-N was lost, compared to just 6% loss from ammonium sulfate. Most of the applied urea was caught in the foliage and thatch of the pasture and did not directly contact the soil, so soil properties had little influence on losses. The urease enzyme is naturally found on plant and litter surfaces so the breakdown of the urea—yielding ammonium and high pH (so more ammonia from ammonium)—begins once it has dissolved. In contrast, the ammonium sulfate would also have dissolved but no high pH is generated, so most stays in the ammonium form.
Most of the fertiliser top-dressed into the wheat crops in our study fell directly into contact with the soil surface as it was applied well before canopy closure. Perennial tropical grass-based pastures have a strong demand for available N during the spring growth period, which rapidly depletes the soil of any ammonium produced. At one of the pasture experiments, most of the applied N not volatilised during the month was found in live pasture biomass, with little left in the soil. The pasture paddocks had negligible soil calcium carbonate, so losses from ammonium sulfate were low and offer a lower risk alternative to urea use for fertilising pastures on Vertosols.
Factors affecting N loss
All non-calcium carbonate (<2%) plot results were pooled together and statistically analysed to test the influence of a range of soil and environmental factors on N volatilisation results (step-wise multiple linear regression). In decreasing order of influence, the main factors determining N loss were; (a) whether the paddock was fallow or in-crop, (b) the type of fertiliser used, (c) the wind-speed at ground level, (d) the fine sand content of the soil, (e) the soil moisture content when fertiliser was applied, and (f) the average temperature in the first week after fertiliser application.
Fallow paddocks typically had greater surface soil moisture at fertiliser application, more rainfall after application and higher average temperatures during the measurement period than in-crop paddocks. Crop canopies can also absorb some of the ammonia volatilized from the soil surface if the ammonia concentration of the air in the canopy exceeds that in the plant leaf. Unless there is a large mass of standing crop stubble, most fallows provide little protection from wind speed at ground level. Increasing wind speed at the soil surface increases the rate of volatilisation by promoting more rapid transport of ammonia-rich air away from the soil surface.
Soil texture was also a significant factor in explaining variation in N loss by volatilisation, with greater losses from sandier soils. As discussed above, this was most likely due to the lower CEC of sandier soils. Soils that were wet when fertiliser was applied were more likely to dissolve the applied fertiliser than dry soils, but as the soil dries out, dissolved ammonia gas is concentrated in the remaining soil water solution increasing the potential for loss.
Recovery of applied N
We measured N volatilised for a period of a month after fertiliser application. At the conclusion of the month, we sampled soils and plants (at in-crop and pasture sites) and measured soil nitrate and ammonium and plant total N. We did not measure N uptake into grain.
At the in-crop sites, most of the N applied was found in the soil nitrate and ammonium, with only a little recovered in the plant since conditions were mostly dry. Similarly, Daniel (2014) reported that most N applied as broadcast urea at GS60 & 77 was recovered in soil samples taken at harvest time.
In contrast, most of the applied N not volatilised from one of the pasture sites we had was found in the living pasture material and little in the soil.
In 3 of the 19 paddocks there was little effective rainfall during the entire month of measurement, so it is likely that some of the N applied as urea was still present as urea and therefore not (yet) subject to loss as ammonia. At 5 other sites, rainfall before the end of the month of measurement delayed soil sampling, leached nitrate lower into the soil profile, and in a few cases waterlogged the soil. Waterlogged soils lose N through nitrate denitrification and significant amounts can be lost. At all other sites, the sum of volatilised N + plant N + nitrate N + ammonium N = applied N, although field variation in soil nitrate and ammonium results was high due to the nature of sampling a large area with a small sampling tube. In comparison, our measurement of ammonia volatilisation came from the whole plot area and therefore incorporated all the variation in soil properties within each plot.
References
Bacon PE, Freney JR (1989) Nitrogen loss from different tillage systems and the effect on cereal grain-yield. Fertilizer Research 20(2), 59-66.
Bouwman AF, Boumans LJM, Batjes NH (2002) Estimation of global NH3 volatilization loss from synthetic fertilizers and animal manure applied to arable lands and grasslands. Global Biogeochemical Cycles 16(2).
Daniel R., Norton R., Mitchell A., Bailey L., and Duncan R. (2014) The effectiveness of nitrogen application for protein – 2012 and 2013. Proceedings of GRDC update, Goondiwindi .
O'Toole P, McGarry SJ, Morgan MA (1985) Ammonia volatilization from urea-treated pasture and tillage soils: effects of soil properties. Journal of Soil Science 36(4), 613-620.
Suter H, Sultana H, Turner D, Davies R, Walker C, Chen DL (2013) Influence of urea fertiliser formulation, urease inhibitor and season on ammonia loss from ryegrass. Nutrient Cycling in Agroecosystems 95(2), 175-185.
Turner DA, Edis RB, Chen D, Freney JR, Denmead OT, Christie R (2010) Determination and mitigation of ammonia loss from urea applied to winter wheat with N-(n-butyl) thiophosphorictriamide. Agriculture Ecosystems & Environment 137(3-4), 261-266.
Turner DA, Edis RE, Chen D, Freney JR, Denmead OT (2012) Ammonia volatilization from nitrogen fertilizers applied to cereals in two cropping areas of southern Australia. Nutrient Cycling in Agroecosystems 93(2), 113-126.
Contact details
Graeme Schwenke
NSW DPI
Ph: 02 6763 1137
Fx: 02 6763 1222
Email: graeme.schwenke@dpi.nsw.gov.au
® Registered trademark
GRDC Project Code: DAN00144,
Was this page helpful?
YOUR FEEDBACK
