Regional movement and sources of canola colonisation by diamondback moth, Plutella xylostella, in southern Australia
Regional movement and sources of canola colonisation by diamondback moth, Plutella xylostella, in southern Australia
Author: Kym Perry, Simon Baxter and Michael A. Keller | Date: 10 Feb 2015
Kym Perry1,3, Simon Baxter2 and Michael A. Keller1,
1School of Agriculture, Food and Wine, University of Adelaide, SA; 2School of Molecular and Biomedical Sciences, University of Adelaide, SA; 3Entomology Unit, SARDI, Adelaide, SA.
GRDC project code: UA00146
Keywords: diamondback moth, colonization, canola, southern Australia.
Take home messages
- This PhD study combines field-based and genetic approaches to identify the sources of seasonal recolonisation of canola crops by diamondback moth in southern Australia, and the implications for management.
- Wild Brassicaceous hosts may maintain over-summering populations of insecticide-resistant diamondback moth on western Eyre Peninsula.
- Canola colonisation by diamondback moth occurred widely during early winter in South Australia in 2014.
- A new cryptic species, Plutella australiana, has been identified by a recent DNA barcoding study.
General background
The diamondback moth (DBM), Plutella xylostella, is the world’s principal pest of Brassica vegetables and a periodic but increasingly damaging pest of canola crops (Furlong et al. 2013). This insect is highly migratory and seasonally recolonises winter canola crops in southern and western regions of Australia. Periodically, DBM outbreaks occur in Australian canola crops during spring, when larval populations rapidly build to extremely high densities, resulting in severe feeding damage and yield losses. Growers are heavily reliant on insecticides to manage outbreaks, however the capacity of DBM to rapidly evolve insecticide resistance is a major challenge to effective control.
An understanding of the ecology of DBM in the canola agro-ecosystem is needed to improve the future management of DBM in canola, however relevant long term ecological datasets do not exist (Furlong et al. 2008). The regional dispersal patterns and sources of DBM populations that seasonally recolonise Australian canola crops are currently unknown, however this information is critical for the development of tools to forecast the risk of outbreaks (Furlong et al. 2008). Furthermore, knowledge of gene flow among DBM populations across regions and host types is needed to assess the risks of resistance spread and devise appropriate insecticide resistance management (IRM) strategies.
New cryptic species, Plutella australiana, revealed by DNA barcoding
Recently, a DNA barcoding study revealed the presence of a cryptic Australian Plutella species, newly described as Plutella australiana (Landry and Hebert 2013). P. australiana moths were detected in light trap catches from various locations in eastern Australia during 2004-2012. The two Plutella species cannot be distinguished based on external morphology, but show clear differences in genitalia and high mitochondrial DNA sequence divergence (8.6%). The seasonal biology, host plants and pest status of P. australiana are currently unknown but need to be identified.
Research aims and methods
The primary research question for this PhD study is: “What are the sources of DBM populations that seasonally recolonise canola crops in southern Australia, and what are the implications for management?” In order to address this question, the study has five related aims (Table 1). In short, the study combines complementary field-based and genetic approaches to elucidate the patterns of regional movement and gene flow among Australian DBM populations, and identify potential sources of seasonal recolonisation of canola by this pest in southern Australia.
Here, a brief overview of the PhD project and key preliminary findings from the first year of research in 2014 is provided. The research continues in 2015 and 2016.
Table 1. Overview of the research approach being used to address the five project aims.
|
Project aims |
Research approach |
|---|---|
|
(i) Assess the extent to which DBM can over-summer locally in canola regions. |
Summer field surveys, sampling and pheromone trapping are being used in conjunction with bioclimatic modelling (CLIMEX) to determine where over-summering is most likely. |
|
(ii) Determine the timing of first canola crop colonisation at a regional scale. |
A regional network of sentinel canola fields is monitored by field consultants from planting to determine timing of first colonisation by DBM. Development models are being used to back-predict the dates of egg-lay (colonisation) in each crop. Geographic patterns in the timing of crop colonisation are being used to infer potential source areas. |
|
(iii) Determine the timing and potential origins of large scale migration flights. |
A regional network of five light traps has been established in canola regions of South Australia. Daily light trap catch data are being used in conjunction with synoptic modelling to identify the timing and potential origins of migration flights. |
|
(iv) Investigate patterns of gene flow among Australian DBM populations. |
New high-throughput DNA sequencing methods are being used to investigate population genetic structure among DBM populations from different hosts and geographic regions across Australia. |
|
(v) Gather information on the seasonal biology and distribution of the cryptic species, Plutella australiana |
Plutella samples from traps catches and field collections are being genotyped to determine the occurrence of P. australiana. |
Selection of preliminary findings from 2014
Over-summering on wild hosts
Field surveys were conducted in the key canola region of western Eyre Peninsula during March and April 2014 to determine the presence of potential DBM host plants and DBM populations. Pheromone traps were placed at 47 locations near wild Brassicaceous host plants in March and left for four weeks. Host plants were sampled for larvae at these locations during March and April.
Lincoln weed (Diplotaxis tenufolia), dog weed (Diplotaxis muralis) and sea rocket (Cakile maritima) were abundant in the region during the sampling period. Sea rocket is a succulent species that commonly occurs in coastal sand dunes. Pheromone traps revealed a low but widespread DBM population across the region during the sampling period (Figure 1A). Larvae were collected from wild hosts in both March and April (Figure 1B). Several larval populations collected from Lincoln weed and sea rocket were subsequently tested for insecticide resistance by SARDI. These populations had similar resistance profiles to populations collected from canola crops in the region, showing resistance to synthetic pyrethroids and organophosphates, and elevated tolerance to Affirm®, SuccessTM Neo and Group 28 insecticides (Baker and Powis, unpublished data). These results suggest that wild Brassicaceous hosts may provide a summer refuge for resistant DBM populations in this region.
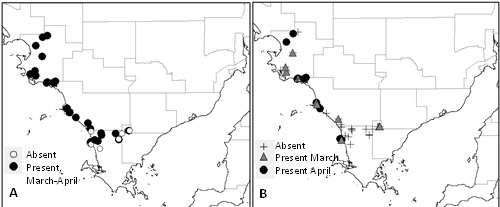
Figure 1: Locations where (A) male DBM moths were detected in pheromone traps near wild hosts, and (B) DBM larvae were collected from wild hosts, on western Eyre Peninsula in March and April 2014
The timing of canola crop colonisation at a regional scale
Little is currently known about the timing of seasonal colonisation of canola crops by DBM, but this information is needed to identify potential source areas and understand the role of local population build-up in spring DBM outbreaks. In 2014, a regional network of 43 sentinel canola fields was established across cropping regions of South Australia and Western Victoria (Figure 3B). Field consultants each monitored 1-2 sentinel fields at approximately weekly intervals from planting to determine the timing of first colonisation of crops by DBM. Pheromone traps were placed in sentinel fields to measure weekly male moth flight activity, and crops sampled for the presence of larvae (signaling colonisation). Data for 33 sentinel fields with reliable data are presented below.
Pheromone trapping data revealed moderate to high moth activity in sentinel canola crops during May to July across most regions (Figure 2). There were regional differences in the magnitude of moth flight activity, most notably between the Upper and Lower South East. 100% of sentinel fields (n=33) were colonised by DBM in 2014. Larvae were first detected in crops as early as May on Upper Eyre Peninsula, and in the majority of canola crops by around July. An exception was the Lower South East, where moth activity was lower than other regions and larvae did not appear until August and September.
In short, these data demonstrate early season canola colonisation by DBM in South Australia in 2014. These results likely reflect early season weather conditions across southeastern Australia that favoured DBM population development, including February and April rainfall that promoted the growth of Brassicaceous hosts, and above-average temperatures in May that promoted population development and flight activity. Subsequent spring populations of DBM in canola reached economically damaging levels in some districts, however the extent to which early crop colonisation contributed to spring population levels is unclear. Monitoring of sentinel fields will be repeated in 2015 to measure seasonal variation in colonisation timing (Please contact the author to participate, contact details at end of paper).
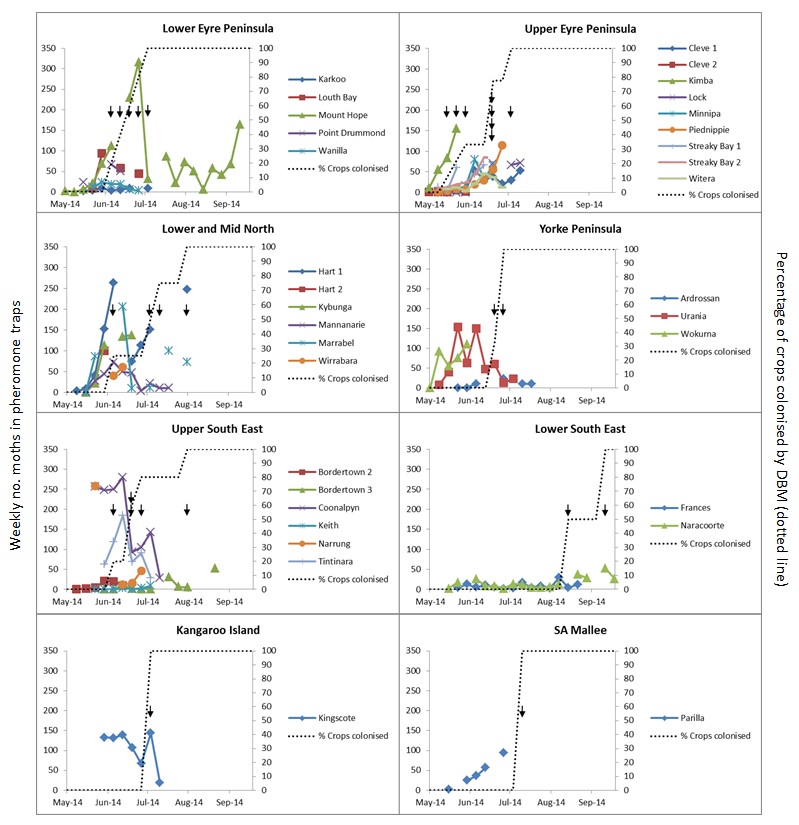
Figure 2. Percentage of crops colonised by DBM in an number of SA locations.
Little is presently known about the seasonal biology and pest status of the newly described cryptic species, Plutella australiana. Fortunately, a simple molecular assay is able to distinguish P. australiana from the diamondback moth, P. xylostella. The assay is being used to determine the occurrence of P. australiana in Plutella samples collected in light traps (Figure 3A) and from Brassicaceous hosts in the field.
Approximately 37% P. australiana was detected in a sample of Plutella individuals (n=16) collected in a light trap at Minnipa, Eyre Peninsula, on 23rd September 2014. No P. australiana was detected in a sample of Plutella individuals (n=63) collected in a light trap at Two Wells, Northern Adelaide Plains, on 17th September 2014. A separate DNA sequencing dataset has also revealed three individuals of P. australiana in a Plutella population (n=8) collected from Lincoln weed at Calca, Eyre Peninsula, on 12th April 2014. These results show that P. australiana occurs on upper Eyre Peninsula in mixed population with the diamondback moth, P. xylostella. The Calca population data also provides the first host record (Lincoln weed) for this species.
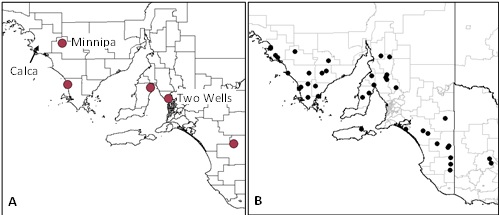
Figure 3: Locations of (A) light trap network in South Australia, and (B) the network of sentinel canola fields monitored to determine the timing of canola crop colonisation by DBM in 2014.
Conclusions and next steps
In its first year, the project has established several lines of research into the patterns of regional movement patterns of DBM in southern Australia. Field observational data gathered in 2014 has so far revealed:
- Wild Brassicaceous hosts may maintain over-summering populations of insecticide-resistant DBM on western Eyre Peninsula.
- Canola colonisation by DBM occurred widely during early winter in South Australia in 2014.
Longer term datasets are needed to measure seasonal variation. Further field studies involving summer surveys, monitoring of sentinel fields and maintenance of a light trap network, are planned in 2015 to generate a second year of field observational data. Field data will be supplemented with phenological and synoptic modelling, followed by detailed analyses. A bioclimatic model (CLIMEX) will be used to predict areas seasonally suitable for population growth. The results of population genetic studies will be presented in subsequent papers.
In addition, molecular assays have revealed the occurrence of the cryptic species, Plutella australiana, on Western Eyre Peninsula in mixed populations with the common DBM species, P. xylostella. The pest status of this cryptic species has not yet been established, but has important implications for DBM management in Australia. This study will continue to gather data on the seasonal biology of P. australiana in collaboration with a new GRDC-funded SARDI study (DAS00155) starting in 2015.
Acknowledgements
We acknowledge the funding support of the University of Adelaide and the Grains Research and Development Corporation (GRDC) for this PhD research. We also gratefully acknowledge the many grains industry advisers, growers and research colleagues who have generously donated their time to assist with collection of field data and biological material during 2014, in particular:
Field data collection (monitoring sentinel canola fields): Sally MacLeod; Peter Ellison, Thomas Cooper, Steve Richmond and Jarrod Kemp (Landmark), Leigh Davis (SARDI), Nigel Myers (Cummins Ag), Andy Bates and Michael Hind (Bates Ag Consulting), Ben Farmer (Alpha Group Consulting), Tim Richardson (Carrs Seeds), Chris Davey (YP Ag), Sarah Traeger and Cindy Martin (Cleve Rural Traders), Clint McEvoy (West Coast Ag), Craig Davis (formerly AW Vater & Co), Craig Wissell (Team Wiss), Dustin Berryman (Northern Ag), Emma Leonard; Hayden Whitwell and Amy Murray (Agsave Kimba), John Haagmans, John Robertson (AgWise Services), Josh Mahoney (Eurofins Research), Lou Flohr (Agrilink), Lyn Dohle (PIRSA), Matt Dare; Michael Camac and Matthew Howell (Coorong Ag Services), Natalie Allen (Jolpac Rural Supplies), Adam Hancock (Elders), Patrick Redden (Rural Directions), Sarah Noake (Hart trials group), Martin Clark.
Light trap operation: James Davey, Peter and Mareike Ellison, Andrew Sharpe, Darren and Jenny; Leigh Davis (SARDI).
Field population collections: Adam Pearce (Clovercrest Consulting), Melina Miles and Adam Quade (QDAFF); Alan Lord, Peter Mangano and Stewart Learmonth (DAFWA), Andy Ryland (IPMC), Chris Davey (YPAg), Craig James; Dustin Berryman (Northern Ag); Greg Baker, Kevin Powis and Michael Nash (SARDI); Peter Ellison, Chris Teague, Greg Dearman and Andrew McMahen (Landmark), Guy Westmore (DPIPWE), Joanne Holloway (NSW DPI), Josh Hollitt (Hollitt Consulting); Levon Cookson, Karina Bennett and Monica Field (Peracto), Lou Flohr (Agrilink), Michael Collins (Elders), Nigel Myers (Cummins Ag), Orville Hildebrand (FP Ag), Peter Cole; Richard Saunders (Dodgshun Medlin), Sarina MacFadyen (CSIRO), Tim Richardson (Carrs Seeds).
Further reading
Furlong, M. J., H. Spafford, P. M. Ridland, N. M. Endersby, O. R. Edwards, G. J. Baker, M. A. Keller, and C. A. Paull. 2008. Ecology of diamondback moth in australian canola: Landscape perspectives and the implications for management. Australian Journal of Experimental Agriculture 48:1494-1505.
Furlong, M. J., D. J. Wright, and L. M. Dosdall. 2013. Diamondback moth ecology and management: Problems, progress, and prospects. Pages 517-+ in M. R. Berenbaum, editor. Annual Review of Entomology, Vol 58.
Landry, J.F., and P. D. N. Hebert. 2013. Plutella australiana (lepidoptera, plutellidae), an overlooked diamondback moth revealed by DNA barcodes. Zookeys:43-63.
Contact details
Kym Perry
(08) 8303 9370; 0421 788 357
GRDC Project Code: UA00146,
