The what where and why of soil testing in the northern region
The what where and why of soil testing in the northern region
Author: Mike Bell and David Lester; University of Qld (Gatton) and DAF (Toowoomba) | Date: 24 Jul 2015
Take home message
Soil testing is a key component of ensuring both sustainable land management in the longer term and maximising the chance of reaching the water-limited yield potential in the coming season. The sampling strategy adopted will be determined by the reason for sampling (fertility monitoring or fertilizer diagnosis), the size and availability of the different pools nutrient in the soil (determining the appropriate laboratory test method), the mobility of the nutrients of concern in the soil profile (determining soil layers of interest) and the root activity of different rotation species in that soil type-seasonal rainfall combination.
The correct soil sampling strategy and diagnosis of potential nutrient limitations will not guarantee an economic response to applied fertilizer, as seasonal conditions and inappropriate application strategy (timing or placement) can reduce the crop nutrient requirement or limit crop recovery of the applied nutrient. However, it will ensure the best possible chance of delivering on water-limited yield potential in the coming season and represents value for money in a farm management plan.
This paper discusses current thinking on soil sampling methodology (frequency, depth intervals), analytical methods and interpretation relative to fertilizer N responsiveness for the northern grains region.
Perhaps the most compelling argument for soil testing is that if you don’t understand the fertility status of the soils under management it is extremely difficult and time-consuming to then ensure the right fertilizer product, application rate, and method of application are used to maximise chances of crop recovery and an economic yield response. This is becoming increasingly important in the northern region as the native fertility levels in our once-fertile clay soils are diminished through grain removal and we become increasingly reliant on external nutrient inputs.
Northern soils and climate
We have some clear advantages in our region over other rain-fed cropping zones. Firstly, moisture stored in the soil profile during a fallow can deliver a significant proportion of our annual crop growth and yield (especially in winter cropping), so once we make a planting decision the questions about crop size (and hence nutrient demand) are more about how much extra growth/yield we may derive from the seasonal forecast of in-crop rainfall rather than whether we will have any crop at all. Secondly, for expensive nutrients like phosphorus (P) and potassium (K), we find these nutrients have an excellent residual value for seasons following the actual application, so we have flexibility to apply these nutrients when our cash flow and seasonal/stubble conditions suit, rather than for each crop. Clearly once we understand the soil status of these nutrients, the different crop species nutrient requirements and the rates of crop removal in harvested produce, we can effectively use nutrient budgeting (fertilizer applied - grain removal) to guide our fertility strategy.
However our heavier soil types also confer some disadvantages, and these relate to the typically mobile nutrients like nitrogen (N – our largest and most expensive nutrient input) and sulphur (S). These nutrients are less mobile in clay soils, and while that means they are less likely to be lost below the root zone by leaching, they are also slower/require higher amounts of rainfall to redistribute into subsoils where much of crop root activity (water and nutrient uptake) occurs. In the case of N, this slow movement into the profile also increases the risk window for significant losses to the atmosphere as a gas through the processes of denitrification and volatilization. These processes predominantly occur at or near the soil surface, although in the case of denitrification, can also extend to deeper layers under prolonged wet conditions. These loss pathways can result in significant losses of plant-available N and so shift the soil nutrient status out of the normal expected range, with implications for fertilizer requirement in subsequent crop seasons. The only practical way to assess the outcome of ‘unusual’ rainfall events/seasonal conditions for these nutrients is soil testing.
Soil testing strategies
Soils can be tested for a range of factors - to estimate how much water can be or has been stored; to identify the depth of root barriers or subsoil constraints such as boron or salinity; or the potential occurrence of a soil-borne disease. In this paper we focus on soil testing in relation to crop nutrition. This testing can be undertaken to either monitor long term fertility trends in cropped fields (i.e. is my fertilizer strategy maintaining my soil available nutrient status, and are these still appropriate to address yield limitations) or to identify the fertilizer requirement for the coming season. The field sampling strategies to address these two objectives are quite different. The first is quite challenging in trying to quantify changes in nutrient status over time, through repeated sampling at the same and at the same (or similar) reference points, to minimize background variability. The second and most commonly applied approach is trying to adequately represent the fertility status across the management unit in question, be it a yield zone, soil type or paddock..
The frequency with which this sampling should be undertaken will be related to the nutrient status of the field (are levels marginal/limiting or is there good background fertility?) and also how quickly the nutrient status can change (in response to crop uptake and removal, or to rainfall events/seasonal conditions). It is important to remember that when interpreting soil test results the values on the report are (i) only as good as the paddock sampling strategy with which they were collected, (ii) have variability associated with the laboratory analysis and detection method, and (iii) are being related to a critical range of soil test values below which a crop response is expected. In other words, normal soil test results should be used as a guide rather than a guarantee, but will still provide a very firm plank in a sensible nutrient management program. Ideally, integrating plant tissue analysis also would provide a more robust assessment of the soil fertility status.
Sampling depths will vary with the nutrient and reflect the zones in the soil profile contributing to meeting crop demands. The most common soil sampling depth for nutrient analysis has been 0 to 10 centimetres for broad-acre crops. This layer was chosen because nutrients, especially P, and plant roots in early growth stages are more concentrated within this layer. However to obtain more comprehensive soil nutrient data, sampling below 10cm should be considered for some nutrients.
Suggested sampling increments for key nutrients (and salinity/sodicity constraints) for northern cropping regions are:
- 0 to 10cm (N, P, K,S and sodicity);
- 10 to 30cm (N, P, K and S);
- 30 to 60cm (N and S, salinity/sodicity);
- 60 to 90cm (N, salinity/sodicity); and
- 90 to 120cm (optional - N, salinity/sodicity).
Deeper sampling does raise issues of logistics and cost, which should be discussed with soil test providers. However, the additional information provides a clearer insight into nutrient status in the crop root zone. Changes in level of nutrient availability or subsoil constraint are very slow so the frequency with which these need to be measured also has longer time scale, amortizing the cost out over many years.
Analytical results and testing methods
Soil test information is most useful for indicating the available amounts of macro-nutrients (those required in relatively large amounts to sustain plant growth – N, P, K, and S, calcium [Ca], magnesium [Mg] and sodium [Na]). Results for micro-nutrients (zinc, copper, manganese, boron) are also useful, but much more as a broad indicator of soil status rather than being directly linked to crop requirements and likely fertilizer response. Tissue testing for micronutrients is typically more informative for plant requirement in that regard.
Appropriate soil tests for measuring soil extractable or plant available nutrients in the northern cropping region are:
- Bicarbonate extractable P (Colwell-P), to assess easily available soil P;
- Acid extractable P (BSES-P), to assess slower release soil P reserves and the build-up of fertiliser residues;
- Exchangeable K;
- KCl-40 extractable S or MCP-S; and
- 2M KCl extractable mineral N, to provide measurement of nitrate-N and ammonium-N.
Tests for N and S provide information on nutrient supply (i.e. they can be directly linked to the quantity of nutrient available to the crop), while P and K tests indicate nutrient sufficiency/deficiency. It should be noted that N (and to a lesser extent S) demand is highly influenced by seasonal conditions, mineralisation from crop residues and soil organic matter between testing and harvest, and crop yield potential, making soil testing for N in isolation an unreliable indicator of fertiliser N requirements.
Other measurements that aid the interpretation of soil nutrient tests include:
- Soil carbon/organic matter content;
- Phosphorus buffering index (PBI);
- Soil salinity measured as electrical conductivity; and
- Chloride and other exchangeable cations (Ca, Mg and Na) including aluminium.
Further details of these analytical methods can be found in the Crop Nutrition factsheet for the northern grains region (http://grdc.com.au/Resources/Factsheets/2014/01/Soil-testing-for-crop-nutrition-North )
Frequency of testing
The frequency of soil testing in a field will be determined by the size of the available nutrient pool, the mobility of each nutrient in soil water and the rates of crop uptake and removal. The availability of nutrients which are accumulated and removed in large quantities (e.g. N), or which are subject to significant loss pathways (gaseous or leaching losses) can change quite quickly, and so will require closer attention. This may include regular soil testing, but under a string of similar climatic conditions, use of a nutrient budgeting approach combined with periodic soil testing can provide satisfactory results. However, as indicated by the problems with N availability after the La Nina years in 2010-2012, once anomalous events occur a soil test re-set is required to quantify the impact and identify the need to change the management approach.
Other nutrients taken up in large quantities but not necessarily removed in grain (e.g. K), and which are not mobile in the soil water, can change their distribution down a soil profile quite quickly, concentrating in shallow topsoil layers. Minimum or no-till management accentuates this nutrient ‘stratification’ so monitoring to detect such changes and develop a management response can be required relatively frequently in soils where (particularly subsoil) nutrient status is marginal.
Noting these exceptions, some general comments about frequency can be considered. Nutrient status in the top 10cm can typically change the fastest due to high root densities, stubble/residue return and fertilizer placement. As a result, these layers are typically sampled with greatest frequency. With the exception of mobile and dynamic nutrients like N, changes in status in deeper layers will be slower, especially in relation to immobile nutrients like P, K and micro-nutrients, and so will require less frequent testing. However an important point is that knowledge about the subsoil nutrient status of each paddock, especially in relation to slow release nutrient pools like BSES-P and limits to root activity like salinity, are essential to allow development of an effective fertilizer management program.
Relating soil test results to likely fertilizer responses
This topic was covered by Chris Guppy in some detail in the 2012 Goondiwindi Updates, and a detailed research program to improve our understanding of the critical soil test ranges below which crop response to applied fertilizer would be expected has been undertaken since then in UQ00063 (PKS in all crops) and UQ00066 (N in sorghum and canola). This work was based on the realization that most attention had been applied to wheat (N responses and the need for starter P based on the 0-10cm layer), with few guidelines for other crops. In the following section we show new information on the relationship between soil test and fertilizer N responsiveness (expressed as % maximum yield with applied N) for sorghum derived under UQ00066, and note that similar relationships have not yet been able to be developed for canola.
We also update (or in some cases simply reproduce) the indicative estimates of critical soil test ranges for P and K reported by Chris Guppy in 2012, and note that results from UQ00063 have yet to resolve the uncertainty around the ability of soil tests to predict responses to applied S – even in a supposedly responsive crop like canola.
Soil test N response for sorghum
The relationship for sorghum (Fig. 1) looks promising for all except low yielding crops, although surprisingly there is no clear indicator of different critical soil profile N contents (below which fertilizer responses are expected) for crops with different yield potentials and presumably N demands – although there are suggestions that lower yielding crops are less N limited when soil profile N at planting is <70-80 kg/ha in the top 120cm. The quantum of sorghum grain yield response to applied N (Fig. 2) increased as profile N reserves at sowing fell, with the rate of increase greater for sites and seasons where crop yield potential was high. These slopes are an indicator of the likely economic benefit of applied N.
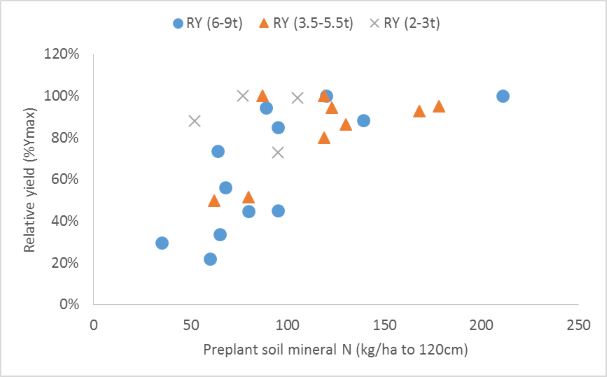
Figure 1. Relationship between relative sorghum grain yield (Y0/Ymax) and profile mineral N (sum of NH4-N and NO3-N) determined in soil tests taken prior to fertilizer application and crop sowing. Relationships are shown for soil profile depths of 120cm, with experiments with different seasonal yield potentials (2-3 t/ha, 3.5-5.5 t/ha and 6-9 t/ha) indicated by contrasting symbols.
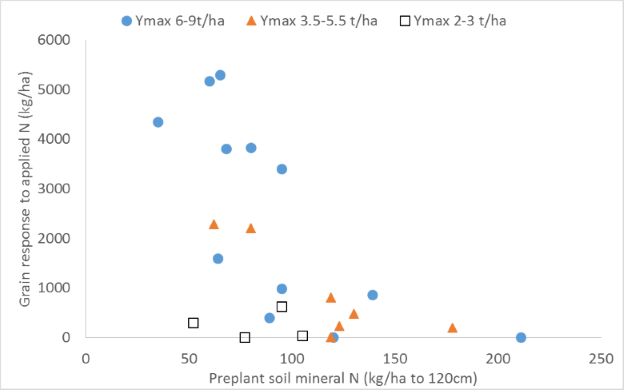
Figure 2. The quantum of sorghum grain yield response to applied N fertilizer (Ymax – Y0) plotted as a function of profile mineral N (120cm depth) at or prior to sowing. The steeper slope of the response surface in sites/seasons with a higher yield potential indicates greater returns on fertilizer N investment.
Phosphorus soil tests
The values listed below for P tests are what we currently use to determine if sites are likely to respond to P (starter P or deep bands), and it is a combination of the two distinct soil P test measurements that give the best indication of likely crop response. Colwell-P is measuring the labile, easily plant available P pool, whilst BSES-P measures not only this pool but also a pool that only releases P very slowly. The key difference is that this slow release pool will not release enough P fast enough to meet the demands of a rapidly growing crop, and so without some rapidly available Colwell P, addition of soluble P fertilizer is required.
Table 1. Generalised critical P values used to determine likely response or drivers of P availability in northern Vertosols
|
Surface (0-10cm) |
Subsoil (10-30cm) |
|||
|
Colwell P |
<25 mg/kg |
Likely to get a response to starter P |
<10 mg/kg |
Likely to get a response to deep P placements |
|
>60 mg/kg |
Ensure good groundcover to limit erosion risk! |
>100 mg/kg |
Unlikely to see P deficiency in your lifetime |
|
|
BSES P |
<25 mg/kg |
Limited evidence of residual fertiliser accumulation |
<30 mg/kg |
Limited reserves of slowly available P. Consider replacement of removed P once every 5 years. |
|
>100 mg/kg |
High residual fertiliser load |
>100 mg/kg |
Potential to slowly replace Colwell P reserves |
|
There are species variations in the critical Colwell values according to species planted. For example maize and wheat require between 25-30 mg Colwell P/kg in the 0-10cm layer, while peanuts require only 12-15 mg/kg, with limited responses above that value. Although we have placed ‘critical values’ in the surface BSES tests in Table 1, we pay very little attention to these values. We are actually content with Colwell and PBI tests in 0-10 and 10-30, and BSES in the 10-30 only, at least once. Because BSES-P releases only slowly, movement in that value takes years, so does not need to be monitored annually.
Because P is an element that roots have to grow towards to maintain uptake, anything that limits the active extension and proliferation of roots will necessarily limit the accessibility of the P that is, at least in a soil test, considered available. Hence, soil conditions that inhibit root growth (sodicity, pH, salinity, nematode damage), necessarily increase the critical values because higher soil solution P concentrations are needed to match demand from a smaller root system. Under these circumstances we would encourage test strips be laid down to determine if remediation is economic. We remain uncertain of the responsiveness of crops to intermediate soil P values, but would expect variation based on the moisture regime the crop experiences each year.
The lower critical values in the subsoil for available P (and available K, below) reflect the larger soil volumes in a 10-30cm depth increment. By the time a plant root system requires nutrients from these depths, many of the yield limits such as grain number have been established in response to early P status (starter P and 0-10cm P status). What is needed by the plant then through to maturity is a long, regular arrival of nutrients from a more extensive and established root system. Plants will only rely on the nutrient status in these subsoil levels when times are hard near the surface. Surface moisture conditions through a season determine the dependence on subsoil nutrient resources, and consequently responses to deep placement. The excellent residual value we have seen from deep P applications in trials in a number of sites suggests that deep P placement followed by a season where topsoil supply dominates does not represent a waste of money. That deep P will be available to subsequent crops in the rotation.
Potassium soil tests
Potassium availability is a little more difficult to establish rules of thumbs for, but Table 2 below summarises our current thinking. Again, there are species differences in these values too. For the majority of species these values are about where we think responses are likely, however, we know that cotton requires higher K availability and critical values in cotton can be almost twice those reported in Table 2.
Table 2. Critical K values used to determine likely response or drivers of K availability in northern Vertosols
|
Surface (0-10cm) |
Subsoil (10-30 cm) |
|||
|
CEC |
ExK (cmol/kg) |
High Mg (>30% CEC) or Na (>6% CEC) |
ExK (cmol/kg) |
High Mg (>30% CEC) or Na (>6% CEC) |
|
<30 cmol/kg |
0.2 |
0.4 |
0.1 |
0.2 |
|
30-60 cmol/kg |
0.4 |
0.7 |
0.3 |
0.5 |
|
>60 cmol/kg |
0.6 |
1.0 |
0.5 |
0.8 |
Considerably more work is required to improve the precision of these critical values, and to understand the mechanism behind the increase in those values where soil Na or Mg status is high. The two main mechanisms are direct competition at the root surface between these cations and K or changes in soil physical structure and aggregation that results in slower root extension and proliferation in the soil volume. It is highly likely that both are important in determining the availability of K to plants, but as yet we are only taking early steps in separating out these effects and understanding which plays a more significant role. Sorting out the importance of each of these mechanisms greatly affects how you manage them, as it will determine whether you attempt to broadcast K widely and enrich a much larger soil volume a little, or concentrate your K in multiple bands at various row spacing. The reason the critical value increases with CEC in Table 2 is because as the CEC of a soil increases, the buffer capacity of the soil for K increases along with it. In essence, the rate at which K is released from the soil to replace that taken up by a plant root is slower than the rate required by the plant root to maintain adequate K status, as the CEC increases. It is very similar to the way a high PBI in a soil increases the critical Colwell P value.
We are continuing to develop a method to estimate the slowly available reserves of K in each soil, with the tetra-phenyl borate extractable K (TBK) method still the most promising. A concerted push to develop testing methods for these slowly available pools is being undertaken by Chris Guppy (UNE) and Phil Moody (DSITI) in the next 3-4 years.
Through our current K field research, whole plant tissue K concentrations at maturity are emerging as a reasonable confirmation of soil K status.
Sulfur soil tests
Critical values for S responses in the surface are currently set at around 6 mg/kg of KCl-40 extractable S and in the subsoil, this would fall to around 4 mg/kg. However, we are currently recommending taking a deeper subsoil test for S, from 30-60cm depth. This is simply because S is far more mobile in the soil profile of heavier clay soils than either P or K, and hence, depending on rainfall, can move vertically in the soil column and be found at deeper depths. Responsiveness to S, where subsoil S is low, is also affected by soil moisture status. A dry topsoil, where organic S reserves accumulate, limits the mineralisation and release of S associated with that organic matter. Often a transient S deficiency can occur in prolonged dry periods, but is relieved with rainfall. At the very least, we are advocating monitoring S levels through the surface 60 cm.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, and the authors would like to thank them for their continued support.
Contact details
Prof Mike Bell
University of Queensland
Plant Science Building (8117A), Gatton Campus
Ph: 0754 601140
Mob: 0429 600730
Email: m.bell4@uq.edu.au
