Fungicide resistance in grain crops
Fungicide resistance in grain crops
Author: Fran Lopez and Alex Kay, Curtin University, Centre for Crop and Disease Management, Department of Environment & Agriculture | Date: 28 Feb 2017
Take home message
- Fungicides are key resources for sustainable farming practices.
- Misuse of fungicides and poor disease management practices have an impact on everybody.
- Overuse of fungicides with the same mode of action will speed up the development of resistance.
- Fungicide resistance is already present in nature but resistant populations get selected and build up under continuous use of fungicides from the same mode of action.
- Resistance is a numbers game and the only viable option to slow it down is to limit the size of pathogen populations. We can do this by use of appropriate rotations, use of clean seeds, early spraying and the use of fungicide mixtures and alternation.
- Fast (and cheap) monitoring of pathogen populations is central for the sustainable chemical management of diseases.
Fungicide resistance overview
Along with cultural practices, the main control measures for the management of fungal diseases are the application of effective fungicides and the use of crop varieties with genetic resistance. However, due to the lack of highly resistant varieties in the grains industry, fungicides are frequently solely relied upon for the control of many fungal diseases. In Australia, the fungicides used against grain diseases are predominantly of the azole or demethylase inhibitor (DMI) group 3, although there are other groups like the quinone outside inhibitor (QoI) group 11 and the succinate dehydrogenase inhibitor(SDHI) group 7 that also contribute to the control as existing mixing partners or new solo and mixed formulations. The threat of so few fungicide modes of action, is that when a fungal disease develops resistance to one fungicide all other fungicides which share the same mode of action, or within the same fungicide group, are also at risk.
So far seven cases of fungicide resistance and two cases of fungicide tolerance in grain diseases have been detected in the last five years in Australia (table 1); many horticultural crops have not been studied yet but anecdotal evidence suggests that many more cases remain to be uncovered.
Table 1. Fungicide resistance cases identified in Australia during the period 2012 – 2017
Disease | Fungicide |
Barley powdery mildewa | DMI |
Wheat powdery mildewa | DMIc, strobilurins |
Canola blacklega, b | MAP/Kinase, DMIc |
Legume Botrytisa | MBC |
Lentil Ascochyta | MBC |
Barley net-blotchesa | DMI |
Wheat septoria leaf blotchb | DMI |
Grape powdery mildewa | QoI/DMI |
Grape downy mildewb | PAA |
Grape Botrytisa | MBC/E1/E3/D1 |
aIdentified by the Fungicide Resistance Group (FRG) in 2015<
aIdentified by the Fungicide Resistance Group (FRG) 2012-2017
bIdentified by other researchers
cTolerance
The azoles remain the backbone of crop protection in broad-acre cropping worldwide and no catastrophic cases of resistance have been reported anywhere in the world. For these reasons, the monitoring of fungicide resistance in Australian agriculture generally was considered unimportant until comparatively recently. Today we can see that the situation in Australia is at a cross-road, with significant levels of resistance in all studied crops.
Resistance in barley powdery mildew starts to develop in the east
DMI resistance in barley powdery mildew was first documented in 2009 in WA. The high levels of resistance detected against certain DMIs were associated with a number of mutations in the target site of this group of fungicides, the most important being mutations Y136F and S509T. Since 2009, great efforts have been made to understand the factors responsible for the rapid onset of this resistance and the spread of the problem (for details see previous paper presented at Bendigo updates titled “From the lab to the field: The scale and impact of fungicide resistance in Australia”), especially to other states across the country.
In 2012, the first of these two mutations, Y136F, was found in barley powdery mildew samples collected from Victoria. This mutation alone has only marginal effects on the sensitivity levels to the fungicides in laboratory conditions, but it is required for the development of further mutations which have been reported to show field resistance. This mutation is therefore what we call a gateway mutation. In 2015, and thanks to the implementation of a new detection technology used in cancer research, the second mutation, S509T, was subsequently detected in NSW, Victoria and Tasmania (figure 1).
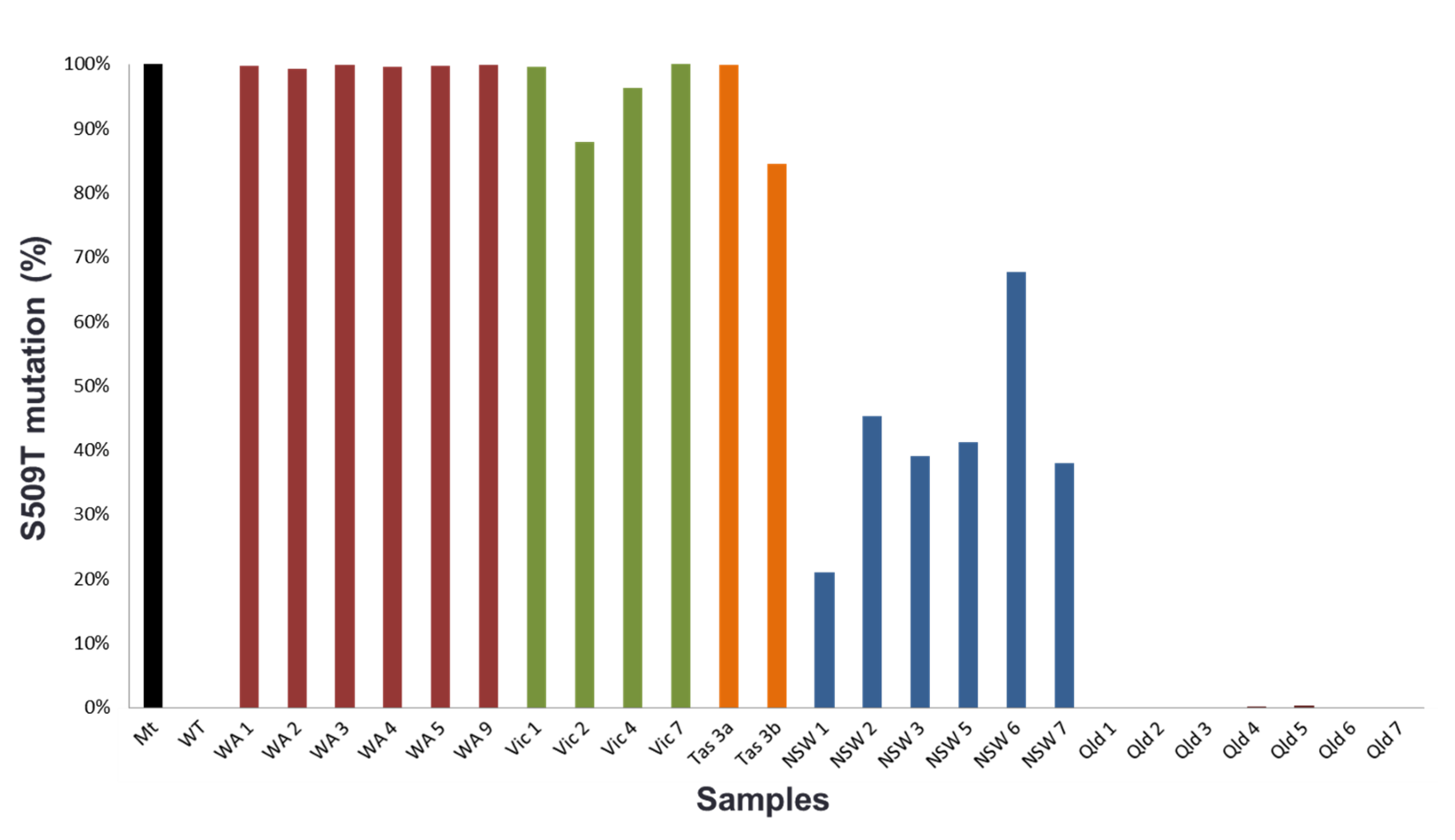
Figure 1. Mutation rates in barley powdery mildew samples quantified by digital PCR in 2016 in Western Australia, Victoria, Tasmania, New South Wales and Queensland. Samples were obtained from barley bait trails specifically developed to catch resistant strains.
In order to limit the potential impact of these mutations spreading across the barley growing areas in the Eastern states, we need to answer the following questions i) is the resistance coming from the West?, and ii) how are the resistant populations going to evolve considering the differences between barley crops in the East and the West in terms of pathogen selection pressure?
The answer to the first question is part of an ongoing research in which the analysis of DNA from both populations tries to determine if there is one centre of origin (West) or if the Western and Eastern fungicide resistant populations evolved and established independently. The second question is probably most important from the disease management point of view. Considering the chemical and cultivar alternatives available, and the existing cultural differences between both areas, it is difficult to envisage a situation similar to the 2009-2012 barley powdery mildew fungicide resistance outbreak recorded in WA. However, in order to reduce the spread of these resistant populations it is necessary to monitor and implement anti-resistance management strategies based on the use of i) chemistries from different modes of actions, ii) varieties with higher levels of resistance, and iii) the introduction of break crops to decrease the disease load from season to season.
Net type net blotch joins the fungicide resistance race
In Australia, emergence of multi-DMI resistance in net type net blotch has been observed in samples collected from the years 2013 onwards. Resistant isolates were geographically widely dispersed across Western Australia, originating in Kojonup, Beverley, Bakers Hill, West Arthur and Dandaragan (Figure 2). We used trials in these studies so as to increase the likelihood of finding resistant isolates even when their frequency is still low. We therefore cannot reliably estimate the frequency of the resistant net type net blotch isolates, but results so far indicate it is at a significant level in Western Australian populations.
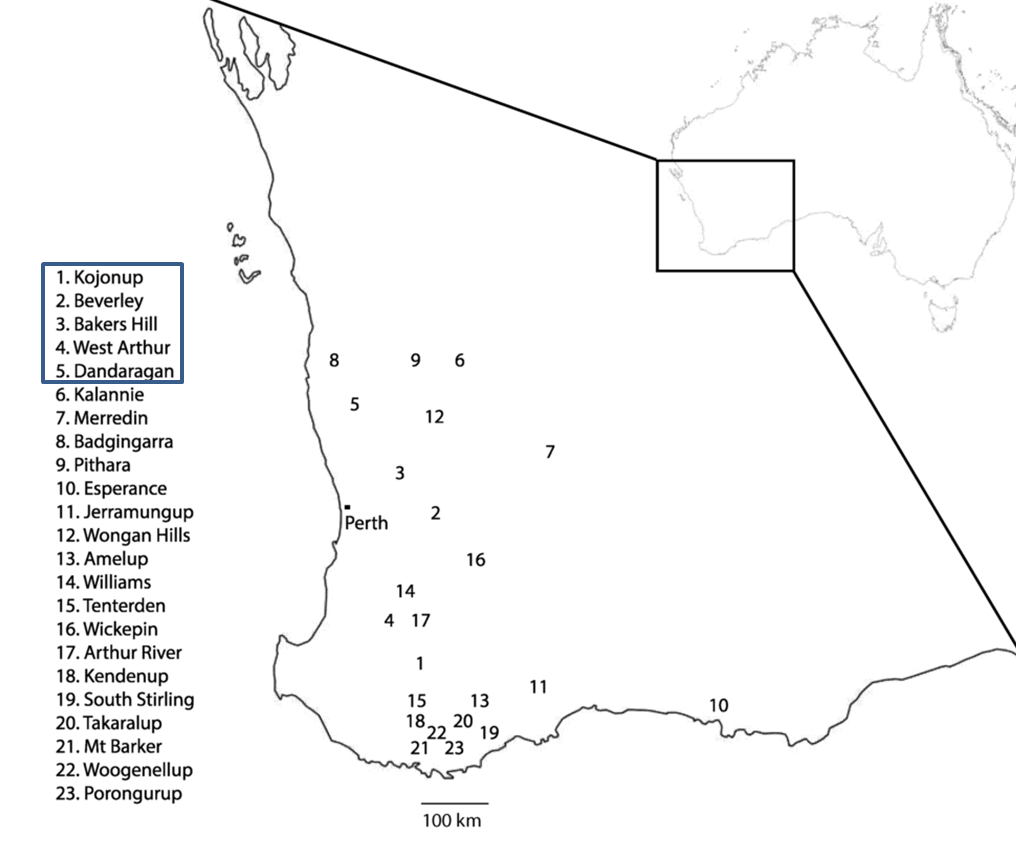
Figure 2. Map of Western Australia showing geographic origin of net type net blotch isolates. Leaf samples were collected from 23 separate locations in Western Australia from a combination of fieldtrips and bait trials. Locations 1, 2 and 14 were specially designed bait trials. Resistant strains were isolated from samples taken in locations 1–5.
When the resistance mechanism was analysed, we found that a combination of mutations and increased expression of the fungicide target were responsible for this resistance in the fungus.
These changes were effectively increasing in vitro the resistance factor (how many times a resistant isolate is more resistant to a given fungicide compared to the average sensitive pathogen population) of the resistant strains when exposed to the fungicides epoxiconazole (RF=1.5), prothioconazole (RF=2.6) and propiconazole (RF=7.7). However, these changes were also increasing the resistance factors for non-registered fungicides such as difenoconazole (RF=12.4), tebuconazole (RF=16.5) and prochloraz (RF=27.7), which critically highlights the fact that fungicides belonging to the same mode of action group (DMIs) are often affected by this type of resistance due to the existing similarities in their structures even if they have not been exposed to the disease previously. So far no resistance has been found in any of the analysed samples from the Eastern States. However, it is important to mention that only a small number of net type net blotch samples were tested and that a large scale analysis is required to determine whether resistance has developed outside of Western Australia.
An important question that remains is the reason why resistance has not developed in spot type net blotch. Net and spot type are two diseases caused by two pathogens that, in addition to the host, share many genetic similarities and undergo similar selection pressures for resistance in the field. We believe that understanding the mechanisms responsible for resistance in net type can contribute to the development of better anti-resistance management practices that can help the industry minimise the risk of fungicide resistance in spot type.
The big question: what is wheat powdery mildew up to?
Wheat powdery mildew has been under the spotlight during the last few seasons due to the increase in field reports claiming lower efficacy of some DMI group 3 fungicides. After a thorough search across the country, in 2015 the gateway mutation Y136F was found in the Eastern Estates but not in WA. As in barley powdery mildew, this mutation has only a marginal effect over resistance but it is necessary for the development of further mutations. In vitro experimental work suggests that the S509T mutation found in barley powdery mildew and responsible for the high levels of resistance against some older DMI fungicides, could develop in wheat powdery mildew as well due to the high similarity between both pathogens. It is then vital to manage the wheat powdery mildew populations carefully so that the development of this concerning mutation is delayed if not prevented.
Unfortunately, and while looking for mutations affecting DMI fungicides, researchers found this year a very important mutation affecting strobilurins (group 11 QoI fungicides) in samples received from Tasmania and Victoria in late 2016. This mutation, named G143A, has been previously described overseas and is associated with a type of cross-resistance that affects all fungicides within group 11. This news will have an important impact in those areas affected by wheat powdery mildew. Formulations containing strobilurins in mixture will be compromised in areas where the resistant populations are found, and their use will potentially add more pressure on the group 3 fungicides. It is recommended to monitor and implement anti-resistance management strategies if growers are planning to grow wheat in mildew prone areas. The fungicide resistance group tests samples for resistance as part of their research activities.
And what about legumes?
With the increase in price for chickpeas and interest building in legumes as crop rotation options, the popularity of legume crops is once again starting to rise. Along with this comes the concern for fungicide resistance development in legume crops, which is fast becoming a hot conversation amongst the grains industry. One concern being raised by chemical sellers is the increase in reliance on fungicides to control Ascochyta blight in chickpea crops, particularly in the northern NSW and southern QLD growing areas where disease inoculum levels are expected to be high from growing chickpeas into infected chickpea stubble. With limited varietal resistance, growers are relying on the control of Ascochyta by fungicides and up to six applications of fungicides have been reported in previous seasons.
So far in vitro resistance to only Ascochyta blight of lentils has been detected to carbendazim a methyl benzimidazole carbamate (MBC) group 1 fungicide (figure 3). Legume botrytis resistance to some MBC group 1 fungicides has also been confirmed. Both cases of resistance have only been detected in samples from South Australia. The research on these two cases of fungicide resistance is limited, and therefore spread of resistance, particularly the presence in other states across Australia, is unknown. However, resistance to strobilurins in Ascochyta blight is well documented overseas and a reminder that this fungal pathogen is capable of developing resistance to different fungicides in Australian legume crops.
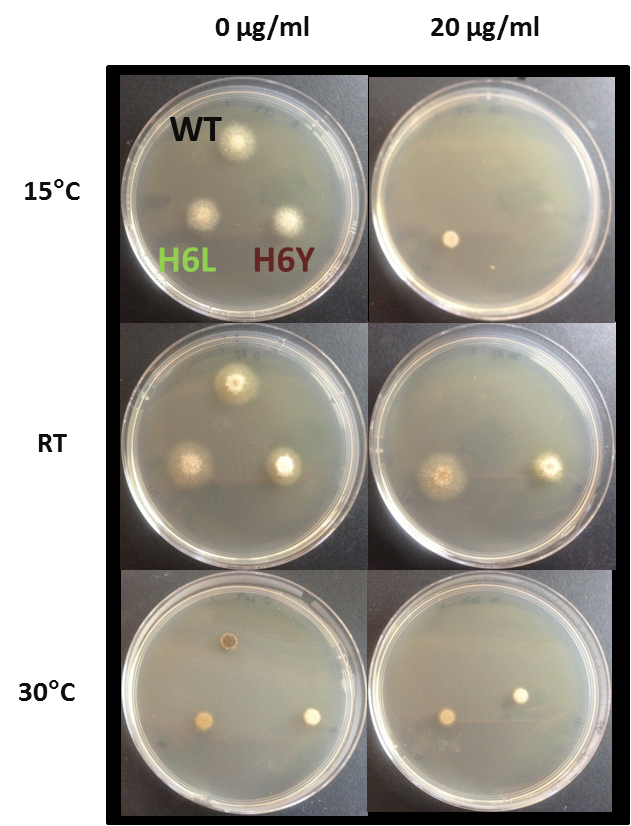
Figure 3. In vitro growth analysis of Ascochyta lentils on two concentrations of carbendazim at three different temperatures. Wild type isolate (WT), mutant H6L and mutant H6Y are labelled on the top left plate.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
The authors would like to acknowledge as well the contribution of Department of Agriculture and Food, Western Australia (DAFWA), The Foundation for Arable Research in Australia (FAR) and the grains industry.
Special thanks go to growers and grower groups submitting samples to the Fungicide Resistance Group (FRG) and contributing to our research.
Contact details
Fran Lopez
Curtin University, Centre for Crop and Disease Management, Department of Environment & Agriculture.
Perth, WA 6845, Australia
Ph: 08 9266 3061
Fx: 08 9266 2495
Email: fran.lopezruiz@curtin.edu.au
GRDC Project Code: CUR00016, CUR00022, DAW00229 and CUR00023,
