Understanding how Sclerotinia sclerotiorum initiates stem rot: factors affecting the germination of sclerotia
Understanding how Sclerotinia sclerotiorum initiates stem rot: factors affecting the germination of sclerotia
Author: Ciara Beard and Anne Smith, DPIRD, Geraldton | Date: 26 Feb 2018
Key Messages
- Under laboratory conditions germination of sclerotia of Sclerotinia sclerotiorum was favoured by a night/day temperature of 10/20°C. None germinated at a higher night/day temperature of 16/29°C and exposure to this high temperature reduced the ability of sclerotia to subsequently germinate when they were moved to a 10/20°C night/day temperature. Understanding the temperatures that favour germination of sclerotia can assist with identifying the potential period in which ascospores may be produced in a given location. In an average year, and assuming sufficient moisture is available, this is likely to be May-October in cooler climates like Esperance, and June-September in warmer climates like Mingenew. Whether this period overlaps with the flowering window of canola crops will determine the risk of stem rot developing in a given season.
- Under a favourable night/day temperature of 10/20°C and ongoing moisture conditions, sclerotia germinated and many produced multiple apothecia (small mushroom like structures) at a time. Some sclerotia were able to produce additional apothecia subsequent to the ones produced when they first germinated and these survived from two to five weeks each over a three month period. This research shows that if favourable weather conditions persist, a steady production of ascospores can be released from one sclerote over several months. Some sclerotia were able to produce additional apothecia subsequent to the ones produced when they first germinated.
- Sclerotia that were ground to simulate the effects of seed destructor technology were still able to germinate under laboratory conditions. Apothecia produced from ground up sclerotia were smaller than those produced from intact sclerotia. Turning the sclerotia into a ‘flour’ (<0.5mm), however, significantly reduced and delayed germination.
Aims
- To further our understanding of how Sclerotinia sclerotiorum initiates the stem rot infection cycle by testing some of the factors that can affect the germination of sclerotia – temperature, soil type, and source of sclerotia.
- To determine if seed destructor technology could offer a method to reduce the ability of sclerotes to germinate.
Method
Two laboratory experiments conducted in 2017 looked at factors that might affect germination and the persistence of apothecia on sclerotia of Sclerotinia sclerotiorum. The first experiment investigated the effect of temperature, soil type and source of sclerotia (location), and the second looked at destruction of sclerotes by grinding them up to simulate seed destructor technology. Germination cabinets with UV lights, 12 hours light and 12 hours dark were used for the experiments.
Sclerotia were sourced from Mingenew and Walkaway (northern wheatbelt) from 2016 lupin harvested seed supplied by growers, and Dalyup (35 km west of Esperance), immediately after 2016 harvest from individual infected canola stems. They were allowed to air dry for six months indoors at room temperature. The aim was to use sclerotia of similar size in the experiment, although the Esperance ones were slightly smaller. Sclerotia were sterilised prior to the experiments as per method used by Hind-Lanoiselet (2006) and allowed to dry. Soils were obtained from paddocks in Mingenew (sandy, pH 7) and Geraldton (loamy, pH 6.5) from areas where canola is regularly sown. Soils were sterilised and dried by placing in an oven at 105°C overnight and then sieved prior to the experiments.
Small (47mm diameter) petri dishes were filled with either sandy or loamy soil and soil moistened by applying 4mL distilled water evenly over soil in each dish. Sterilised sclerotia were placed on soil surface. The petri dishes were placed in an alfoil tray that had a layer of moistened filter paper in it, the alfoil tray placed into a zip lock plastic bag and sealed. There were three petri dishes placed in each alfoil tray. Dishes were watered twice a week initially and then once a week and filter paper kept moist. Photos were taken of each dish and germinations recorded before watering each week. A sclerote was considered germinated if it produced a stipe (the stick of the ‘mushroom’ which in most cases continued on to produce a top, apothecia). Trays were randomly rearranged in the cabinet after watering each week.
Experiment 1: Temperature, soil type, and source of sclerotia
Two germination cabinets were used with temperature 10/20°C night/day (10°C in dark and 20°C in light) in one cabinet to simulate Esperance late summer/autumn temperatures, and 16/29°C night/day (16°C in dark and 29°C in light) in the other cabinet to simulate Mingenew late summer/autumn temperatures. The experiment commenced on 11/05/17 and was completed on 01/11/17.
Treatments 1-4: sclerotia from Mingenew and Esperance on both sandy and loamy soil types were placed in the 10/20°C temperature cabinet for the duration of the experiment.
Treatments 5-8: sclerotia from Mingenew and Esperance on both sandy and loamy soil types were placed in the 16/29°C temperature cabinet for 78 days, and then they were moved to the 10/20°C temperature cabinet for the remainder of the experiment (97 days).
Each petri dish was one soil treatment and housed one source of sclerotia. Five sclerotia were placed in each petri dish in distinct locations so that germination for each could be monitored and recorded over the experiment. Each treatment was replicated 6 times.
Experiment 2: Simulating seed destructor technology by grinding sclerotia
A large quantity of sclerotia (sourced from Walkaway) were ground up in a coffee grinder and then sieved to four different size fractions : <0.5mm, 0.5-1mm, 1-2mm, and >2mm. For each size fraction, 6 petri dishes were lined with soil (Geraldton loamy soil, pH 6.5), divided into quarters and an equal amount of each size fraction was placed in each quarter of the petri dish which equated to 0.01 g of the finest three fractions and four pieces of sclerote for the >2mm grind. With 6 petri dishes and 4 locations on each petri dish this gave a total of 24 reps for each size fraction.
The petri dishes were placed in the 10/20°C temperature cabinet for the duration of the experiment. The experiment commenced on 19/10/17 and is still ongoing. Results are presented for the first 92 days until 18/1/18.
Results
Experiment 1: Temperature, soil type, and source of sclerotia
Across all the treatments, 74% of the sclerotia that were tested germinated. Of those, many sclerotia produced multiple apothecia, and apothecia generally persisted for at least 3 weeks, range was 2 – 5 weeks. Some sclerotia were able to produce additional apothecia subsequent to the ones produced when they first germinated. For example, the sclerote that produced the most apothecia produced 15 over a three month period. With the naked eye, apothecia were observed releasing spores in many treatments when the trays were opened to water them (due to a change in humidity). Some apothecia were observed to produce multiple releases of spores over the three week period that they survived.
Temperature
Of the two temperatures tested (under continuing moist conditions), 10/20°C (night/day temperature) was found to be the optimal one for the germination of sclerotia from both Mingenew and Esperance with the number of days to sclerote germination being on average 59 days (ranged from 36 – 131 days, median was 54 days) with 81% of sclerotia germinating. No sclerotia in the higher temperature (16/29°C) had germinated after 78 days. These sclerotia were moved to the 10/20°C temperature for the remainder of the experiment where some germinated 19 days later; the number of days to germination ranged from 19-68 days (average was 33, median was 30 days), and 68% of sclerotia germinated. Exposing the sclerotia to the high temperature (with moisture) for 78 days reduced their ability to germinate in all treatments except for the loamy soil where the difference was negligible (Table 1).
Soil type
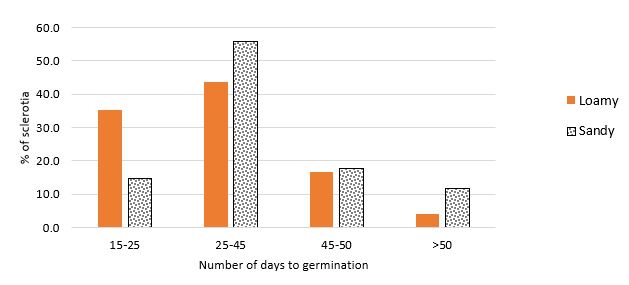
In this experiment where soil moisture was maintained, there were on average more apothecia produced per sclerote on the loamy soil than there were on the sandy soil in both temperature treatments (Table 1). However, soil type did not influence the time taken for sclerotia to germinate in the continuous 10/20°C temperature. For the sclerotia at 16/29°C, when they were moved to 10/20°C, the sclerotia germinated more quickly on the loamy soil than they did on the sandy soil (average of 31 days, compared to 36 days, LSD 10% = 3.4), Figure 1.
Source of sclerotia
There was some variability in sclerote germination depending on the region of the wheatbelt and/or crop that the sclerotia were sourced from. In both the temperature treatments, Esperance sclerotia (sourced from a canola crop) produced more apothecia per sclerote than the Mingenew sclerotia that were sourced from a lupin crop (3.2 apothecia/sclerote for Esperance and 2.4 apothecia/sclerote for Mingenew, LSD 10% = 0.6).
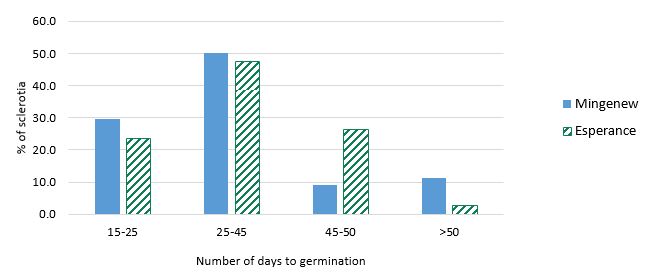
In both temperatures, more Mingenew sclerotia germinated in the first 50 days of the experiment than Esperance ones (for example in Figure 2, 80% of Mingenew sclerotia had germinated by 45 days, whereas only 71% of Esperance sclerotia had germinated by this point). Mingenew sclerotia took longer to germinate a second generation of apothecia than Esperance sclerotia, averaging 117 days compared to 112 days (LSD 5% = 3.1). Exposing the sclerotia to the high temperature (16/29°C night/day) for 78 days particularly reduced the ability of the Mingenew sclerotia to subsequently germinate in 10/20°C (Table 1).
There were no significant soil x sclerote source (location) relationships observed in either temperature treatment.
Table 1: Percentage of sclerotia that germinated in each treatment of Experiment 1. Temperatures are night/day temperatures.
Treatment | Percentage of sclerotia that germinated in each treatment | Average number of apothecia produced per sclerote in each treatment | ||
|---|---|---|---|---|
Continuous | 16/29°C (78 days) | Continuous | 16/29°C (78 days) | |
Loamy soil | 89 | 88 | 3.3 | 2.9 |
Sandy soil | 92 | 81 | 2.5 | 2.4 |
Mingenew sclerotia | 93 | 84 | 2.7 | 2.1 |
Esperance sclerotia | 88 | 84 | 3.1 | 3.2 |
Experiment average | 81 | 68 | 2.9 | 2.6 |
Figure 1: Of the sclerotia that did germinate, this graph shows the number of days they took to first germinate on loamy and sandy soil, 15-25, 25-45, 45-50, >50 days after the plates were moved from 16/29°C to 10/20°C (night/day temperature).
Figure 2: Of the sclerotia that did germinate, this graph shows the number of days for those from Mingenew and Esperance to first germinate, 15-25, 25-45, 45-50, >50 days after the plates were moved from 16/29°C to 10/20°C (night/day temperature). Shown as percentage of sclerotia that did germinate.
Experiment 2: Simulating seed destructor technology by grinding sclerotia
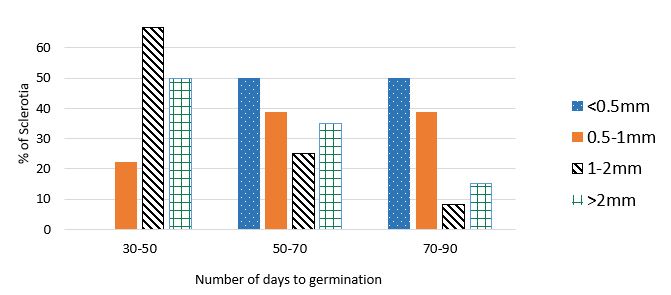
Sclerotia germinated in all treatments despite being ground to different degrees. Each quarter of the petri dishes was counted as germinated if at least one apothecia grew on it. The most effective at reducing the ability of sclerotia to germinate was the finest grind (<0.5mm) (Table 2) and this delayed germination (Figure 3). The 1-2mm grind appeared to encourage earlier and higher rates of germination as well as more apothecia than the other size fractions (Table 2, Figure 3).
It was noted that the apothecia that formed on all the ground sclerote treatments were smaller in size (estimated at 1-2.5mm) than those that formed on intact sclerotia in Experiment 1 (about 5mm diameter). Apothecia size appeared to be closely related to the size of the fraction, with their size decreasing as the sclerotia were ground more finely. Apothecia that grew on the 0.5-1mm fraction did not persist as long as those on the larger size fractions (Table 2).
Table 2: Average days for apothecia to be produced and germination percentages for sclerote ground fractions. Results are presented for the first 92 days of the experiment until 18/1/18, it is ongoing. *Note finest fraction (<0.5mm) had a sample size of only 2, as very few have germinated to date.
| Per Petri-dish quarter (24 replicates of each fraction) | |||||
|---|---|---|---|---|---|---|
Sclerote ground fraction (size) | Average number of days to start germinating | Average number of days apothecia survived for | % Germinated | Average number of apothecia produced | Percentage producing > 1 apothecia (%) | Percentage producing 3 or more apothecia (%) |
< 0.5mm * | 73 | 10 | 8 | 1 | 0 | 0 |
0.5-1mm | 65 | 16 | 75 | 1.8 | 33 | 17 |
1-2mm | 50 | 28 | 100 | 2.7 | 92 | 54 |
> 2mm | 56 | 29 | 83 | 1.4 | 21 | 4 |
LSD (5%) | 5.5 | |||||
Figure 3: Graph showing the number of days for apothecia to first appear in the petri-dish quarters of the sclerote ground fractions <0.5mm, 0.5-1mm, 1-2mm, and >2mm in Experiment 2, 30-50, 50-70, and 70-90 days after the experiment commenced in 10/20°C (night/day temperature). Shown as percentage of sclerotia that did germinate. Note finest fraction (<0.5mm) had a sample size of only 2, as very few have germinated to date. Results are presented for the first 92 days of the experiment until 18/1/18, it is ongoing.
Conclusion
The results of the temperature experiment are helpful to understand the epidemiology of the disease in different regions of the WA wheatbelt. The indication is that average temperatures on the south coast of WA would be conducive to sclerotia germinating from May – October when temperatures are in the 10-20°C range, as long as there was also sufficient moisture. In the northern wheatbelt however, temperatures are only likely to favour sclerote germination from June – September if there is sufficient moisture. Based on the results of these experiments it is suggested that in Esperance, sclerotia could start germinating and spreading ascospores in May although if canola crops don’t start flowering until July, the crops might miss the window of infection if early apothecia don’t persist that long.
In Mingenew, however if sclerotia start germinating in May then spore showers are more likely to overlap with the flowering window which starts in July. The days taken for sclerotia to germinate in these experiments should not be used as a guide for what happens in the field. It is almost impossible to know what level of moisture a sclerote in the field has received, whereas these experiments were conducted with completely dry sclerotia and in germination cabinets which will not reflect the precise light/humidity present under a canola canopy in a field situation.
Exposing sclerotia to higher temperatures than their optimal (such as 16/29°C) may reduce their ability to germinate, though in this experiment this occurred in conjunction with ongoing moisture which could be a key factor. Further work into sclerote management options that encourage sclerotia to be exposed to the heat on the soil surface or burnt in windrows (as opposed to burying them or leaving them protected in standing stems after harvest) would be useful.
These experiments demonstrated how hardy and prolific sclerotia can be. Considering that it is known that each apothecia is capable of producing millions of ascospores, these results demonstrate the potential for infection even if only a few sclerotia are present in a paddock. The previous history of a paddock in terms of disease presence is thus a key factor to consider when planning crop rotations and disease management strategies.
All four sclerote ‘grinds’ germinated, but the finest grind (<0.5mm) showed very low germination to date and germination was significantly delayed. Unless seed destructor technology can consistently grind sclerotia to less than 0.5mm this would not be an effective strategy for reducing the viability of sclerotia in a paddock. However, use of seed destructor technology may reduce fecundity as apothecia on ground up sclerotia were significantly smaller than those produced by intact sclerotia and did not persist for as long, so consequently may release fewer spores. Further research is warranted to determine if smaller apothecia release fewer spores than larger apothecia. It is also acknowledged that grinding up sclerotia may make them more vulnerable to being eaten by mice or ants or broken down by bacteria, which was not investigated in this current work.
Acknowledgments
For guidance on the laboratory techniques to use, we acknowledge the PhD thesis of Tamrika Louise Hind-Lanoiselet titled “Forecasting Sclerotinia stem rot in Australia.”
For supplying us with sclerotia, we wish to thank - Andrea Hills (DPIRD, Esperance), and members of the Mingenew Irwin Group.
For assisting us with setting up some of the experiments, we thank Bonnie Jupp (DPIRD).
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and GRDC investment, the authors would like to thank them for their continued support.
GRDC Project Numbers: UM00051, DAW00228
References
Hind-Lanoiselet T (2006) Forecasting Sclerotinia stem rot in Australia Thesis (PhD)—Charles Sturt University