Herbicide residues in soil – what is the scale and significance?
Take home messages
- Herbicides including trifluralin, 2,4-Dichlorophenoxyacetic acid (2,4-D), diuron, glyphosate and diflufenican were detected in soils in more than 30% of paddocks surveyed prior to planting.
- Herbicides, when applied at label rates, do not cause significant impacts on soil microbial functions. In particular, glyphosate, even with repeated application over time, had no significant deleterious effect.
- There are a small number of examples where herbicide residues detected at planting exceed toxicity thresholds for the crop. Some of these thresholds have been confirmed in laboratory assays.
- Tenosols (light textured sandy soil) are considered at greatest risk of crop damage from residual herbicides due to their lower capacity to bind herbicide, therefore rendering a greater proportion of the residual herbicide as bioavailable.
- Growers need to carefully adhere to recommended plant back periods for sensitive crops and be especially careful if the seasons have not lent themselves to conditions suitable for complete herbicide breakdown. Carryover can result in reduced nitrogen fixation in a following legume crop.
Background
Increasing herbicide use over the last two decades has led to concerns over the potential effects herbicides (and their residues) have on soil health. There is some uncertainty as to whether there is a risk that herbicide residues are accumulating in soils, particularly in low rainfall environments. Risks include chronic low-level yield losses and reductions to profitability, or on the other hand, the perceived risk may be leading to decisions such as variety or crop selection which limits returns. This project was conducted to resolve the question of whether increased herbicide use has negative impacts on soil biological functions, and to benchmark levels of herbicide residues in soil at sowing to determine the possible extent to which they are responsible for causing crop damage and yield decline.
Methods
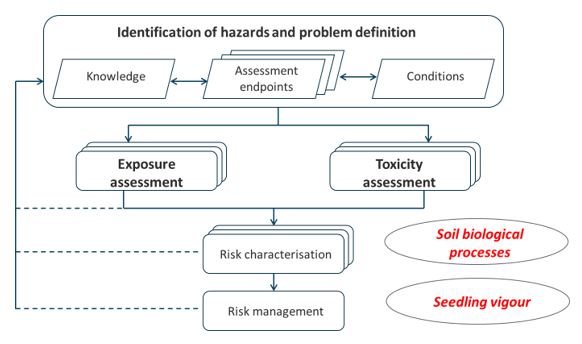
A risk assessment framework was used to assess the potential extent of soil and crop health decline due to herbicide residues across the grains industry (Figure 1). This requires a determination of exposure; i.e. how much herbicide is the soil/crop being exposed to, and toxicity; i.e. what is the residue level that reduces soil function (e.g. nitrification) or plant growth (e.g. shoot biomass) by 20%.
Figure 1. Risk assessment framework used to assess the potential scale of reduced soil and crop health due to herbicide residues in soil.
Exposure to herbicide residues was determined by conducting two field surveys of herbicide residues in soil at sowing. The first survey in February 2015 to April 2015 analysed samples from 40 paddocks around Australia at two depths, 0-10cm and 10-30cm. The second survey used a subset of samples from 40 paddocks within the National Paddock Survey (BWD00025), in which composite samples were taken from 0-10cm from two different zones in each paddock. Samples were analysed by multiresidue techniques, using targeted extraction and liquid chromatography with mass spectrometers (LC-MS/MS) and gas chromatography in combination with mass spectrometry (GC-MS) analysis. Note that extraction methodologies were optimised to determine the total soil concentration of herbicides rather than the bioavailable fraction.
Toxicity to soil biological functions was determined through meta-analysis of the published literature and laboratory soil incubation experiments. Information extracted from over 340 peer-reviewed journal articles was compiled to identify and rank herbicides according to toxicity to soil biological functions, including carbon turnover, nutrient cycling and disease suppression. Literature findings were validated under Australian soil conditions by applying seven commonly used herbicides (glyphosate acid, 2,4-dichlorophenoxyacetic acid [2,4-D], metsulfuron-methyl, trifluralin, diuron, atrazine and diflufenican) and one fungicide (tebuconazole) to five contrasting cropping soils at a recommended and five times recommended rate. Soil functionality was assessed using a range of tools including multi-enzyme (e.g. β-N-acetylglucosaminidase and leucine aminopeptidase contributing to organic N transformation), substrate-induced respiration techniques and the nitrification assay.
An experiment was also conducted to determine the potential effects of repeated applications (1, 3 or 9 doses) of glyphosate at 2.2 kg a.i./ha to three contrasting soil types over a period of 10 months. Microbial community structure was determined at the end of the incubation by next-generation sequencing of 16s ribosomal RNA (rRNA) and internal transcribed spacer (ITS) regions for bacteria and fungi, respectively.
In order to assess the relevance of soil borne herbicide residues on crop growth, international literature was accessed and compiled to identify toxicity thresholds. To meet required quality criteria, the work needed to include a dose-response curve, where a crop was sown into soil with increasing herbicide concentrations, and a shoot or root growth response measurement (either length or biomass). Search terms included ‘herbicide’ and ‘soil’ and ‘phytotoxicity or bioassay’ and ‘crop’ or ‘plant’, where iterative searches were conducted using the specific herbicide as a search term. Where relevant papers were found, references and citations of those papers were checked for additional relevant papers not picked up by the original database searches. To validate literature data (trifluralin, sulfonylureas) or provide missing data (clopyralid), dose-response bioassays were conducted for soil borne trifluralin phytotoxicity to wheat, and trifluralin, metsulfuron-methyl and clopyralid phytotoxicity to lupins. The soil used was a sandy Tenosol from Wongan Hills, Western Australia, with low organic matter. This represented a ‘high-risk’ cropping soil due to its low herbicide sorption and low microbial activity hence slower herbicide degradation. Increasing doses were applied to soil one month before sowing and soil was analysed for herbicide residue level at sowing. Shoot biomass was measured 18 days after sowing. The effective dose required to reduce shoot biomass by 20% (ED20) was calculated by fitting log-logistic response curves to each data set. Due to the lack of data from literature meta-analysis, toxicity thresholds were pooled for monocots (oat, wheat, barley) and dicots (lupin, lentil, field pea, canola) and the geometric mean of the ED20 for each crop type was used as an estimated ‘average’ threshold. Hazard assessments were performed by comparing herbicide dose-response thresholds (toxicity) to residue survey data (exposure) and qualitatively characterising sites where toxicity exceeded exposure.
Results and discussion
Exposure assessment – benchmarking herbicide residue levels in soils
Results for the 2015 and 2016 soil survey demonstrated similar trends of herbicide residues in soil just prior to planting, despite being undertaken on different paddocks, taken by different staff and in different years. Report levels from the 2016 survey are reported here, with results from 2015 presented in a previous update paper (Rose et al., 2016). As with the 2015 survey, glyphosate and aminomethylphosphonic acid (AMPA) were frequently detected (67% and 93% of samples, respectively), with similar median concentrations of 218µg/kg and 308µg/kg, respectively. In 2016, the most frequently detected herbicide (in 94% of all samples) was 2,4-D; but as with the 2015 survey, 2,4-D concentrations were generally low, with 75% of samples containing <3µg/kg (i.e. <1% of a conventional application dose). Trifluralin was also frequently detected (>50% of samples) with similar 75th percentile values to 2015, but with a substantially higher maximum residue concentration of 5345µg/kg in 2016 compared to 590µg/kg in 2015. Diflufenican, MCPA and diuron were also detected in 30% or more of the 2016 samples. Of the additional herbicide residues screened in 2016 that were not analysed in 2015, pyroxasulfone and metolachlor were both detected in 18% of samples, with maximum concentrations of 27µg/kg and 60µg/kg, respectively.
Table 1. Concentration of herbicide residues in 0-10cm soil samples taken prior to sowing (March-April) in 2016.
Group | Active | Detection Frequency (%) | Median concentration (µg/kg) | 75th Percentile concentration* (µg/kg) | Maximum concentration (µg/kg) |
|---|---|---|---|---|---|
A | Clethodim | 5 | 0 | 0 | 14 |
B | Triasulfuron | 12 | 0 | 0 | 3.3 |
Metsulfuron-Methyl | 4 | 0 | 0 | 0.6 | |
Sulfometuron-methyl | 0 | 0 | 0 | 0.0 | |
Chlorsulfuron | 4 | 0 | 0 | 0.7 | |
C | Simazine | 13 | 0 | 0 | 40 |
Atrazine | 6 | 0 | 0 | 25 | |
Terbuthylazine | 5 | 0 | 0 | 29 | |
Metribuzin | 2 | 0 | 0 | 6 | |
Diuron | 30 | 0 | 12 | 275 | |
D | Trifluralin | 51 | 4 | 95 | 5345 |
F | Diflufenican | 60 | 12 | 20 | 137 |
I | MCPA | 42 | 0 | 0 | 66 |
Dicamba | 0 | 0 | 0 | 0 | |
2,4-D | 94 | 1 | 3 | 107 | |
Fluroxypyr | 4 | 0 | 0 | 1 | |
Triclopyr | 26 | 0 | 0 | 34 | |
Clopyralid | 5 | 0 | 0 | 6 | |
J | Prosulfocarb | 7 | 0 | 0 | 28 |
K | Pyroxasulfone | 18 | 0 | 0 | 27 |
Metolachlor | 18 | 0 | 0 | 60 | |
M* | Glyphosate | 67 | 218 | 588 | 3640 |
AMPA | 93 | 308 | 615 | 2270 |
* i.e. 25% of samples contained residue levels above the concentration shown in this column
Toxicity assessment – soil functions
A review of over 340 peer-reviewed articles found that there is little evidence for consistent, long-term impacts to soil (microbially-mediated) functions caused by herbicides when used at registered label rates. Some site-specific exceptions include the interaction of sulfonylurea herbicides with certain pathogens (e.g. rhizoctonia) causing greater disease risk as well as inhibition of N-cycling on alkaline soils. Our controlled laboratory experiments screened the impacts of seven different herbicides (glyphosate, metsulfuron-methyl, 2,4-D, atrazine, diuron, trifluralin, diflufenican) on soil enzyme activities and nitrogen (N)-cycling in five different soil types and confirmed that effects are minimal at maximum label rate application. Application over label rate (5 times) of metsulfuron-methyl had significant but minor impacts (<25% of control level) on N-cycling in three of the five soils tested (impact on two alkaline soils and one low OM soil). In a subsequent nine-month incubation experiment, single or repeat application of glyphosate at 2.2kg a.i./ha every three months at label rates had no significant effects on soil microbial communities or their function, across the three different soil types (Table 2). Monthly application of glyphosate only caused negative impacts in the Tenosol soil type (sandy, low organic matter) but not the heavier-textured Chromosol or Vertosol soil type (Table 2).
Table 2. Effect of repeated dose of glyphosate (as Roundup CT®) over 10 months on soil biological functions.
Glyphosate application over the 10-month incubation | Chromosol | Vertosol | Tenosol |
|---|---|---|---|
1 dose at start | No significant effect | No significant effect | No significant effect |
1 dose at end | No significant effect | No significant effect | No significant effect |
3 doses | No significant effect | No significant effect | No significant effect |
9 doses | No significant effect | No significant effect | Arabinose (¯ 15%) Glucose (¯ 15%) Cellulase (¯ 30%) Phosphatase ( 25%) Chitinase ( 25%) |
Toxicity assessment – crop biomass/vigour
Despite reviewing over 250 peer-reviewed or publically available documents, only a small number of relevant data could be obtained to determine the threshold soil concentrations of herbicides that cause crop phytotoxicity. The majority of these were for the sulfonylurea herbicides, mainly because bioassay techniques were previously the most sensitive method for detecting residues. Sulfonylureas can still be biologically active against dicotyledonous crops at levels near the limit of detection of chemical analysis techniques, with an estimated average ED20 at 0.2µg/kg. There were a useful number of threshold values also available for trifluralin and the triazines simazine and atrazine (Table 3), but a significant knowledge gap for many herbicides detected in the residue survey; including diuron, diflufenican, pyroxasulfone, metolachlor and group I herbicides remains. This paucity of knowledge is a significant drawback in the interpretation of the practical implications of soil residue data.
Table 3. Dose-response thresholds (ED20) for 20% reduction to crop growth (either root or shoot) in short-term bioassays (<28 day). Values are from numerous literature sources and averaged (geometric mean) across plant types. Dicotyledonous crops include lentil, field pea, lupins, canola, chickpea, mungbean and sugarbeet. Monocotyledonous crops include oats, wheat and barley.
Group | Active | Estimated average ED20 for Dicotyledonous crops (µg/kg) | Number of data points obtained | Estimated average ED20 for Monocotyledonous crops (µg/kg) | Number of data points obtained |
|---|---|---|---|---|---|
A | Clethodim | NA | NA | ||
B | Sulfonylureas | 0.2 | 40 | NA | |
C | Triazines | 160 | 14 | 60 | 10 |
Diuron | NA | 900 | 1 | ||
D | Trifluralin | NA | 130 | 8 | |
F | Diflufenican | NA | NA | ||
I | Phenoxys | NA | NA | ||
Triclopyr | NA | NA | |||
Clopyralid | 50 | 1 | NA | ||
J | Prosulfocarb | NA | NA | ||
K | Pyroxasulfone | NA | NA | ||
Metolachlor | NA | NA | |||
M* | Glyphosate | >1200 | 5 | >1400 | 2 |
AMPA | NA | NA |
*Although thresholds are soil type-dependent for all herbicides, the relatively high variability in glyphosate bioavailability across soil types makes it difficult to ascribe a single threshold value. The value given is the lowest observed threshold; occurring for lupin (dicot) or wheat (monocot) growing in a sandy soil with banded phosphorus (P) fertiliser. NA = no suitable data found from the review of public literature.
Hazard assessment – crop biomass/vigour
Taking into account the lack of threshold data available for many of the herbicide residues detected, a hazard analysis was performed for glyphosate, trifluralin and the sulfonylurea herbicides, for which adequate thresholds were available. For glyphosate, only three paddocks from the 40 analysed contained residues that would potentially impact upon legumes grown in Tenosol with P fertiliser (Figure 2A). Of these, two were cropped with cereals, which are much more tolerant to glyphosate residues, even when P is applied, and are unlikely to have suffered injury. Previous work (Rose et al., 2018) has shown that the co-application of banded P in particular can increase the availability of glyphosate in soil as is competes for similar binding sites and allows for greater phytotoxicity. The tolerance of vetch is unknown. For trifluralin, three paddocks contained residues that could potentially injure the cereal crop sown that season, two of which were in WA and one in Vic (Figure 2B). Whether or not some early damage eventuated would depend on where these residues were located within the profile in relation to the placement of the seed, and the influence of soil type on the bioavailability of the residues. The lighter-textured WA soils are not expected to bind the trifluralin as well as the heavier-textured Victorian soil, and therefore, these soils are more likely to see potential crop damage. For sulfonylureas, seven out of the 40 paddocks sampled contained residues that could affect legume crops (Figure 2C). Of these, two paddocks were planted with lentils, one of which was PBA Hurricane variety, which exhibits some tolerance to sulfonylurea (SU) residues. Overall, there was a small number of paddocks with potentially phytotoxic residues, which may limit flexibility of crop selection, but in the majority of cases the potential damage appears to have been avoided by planting tolerant crops.
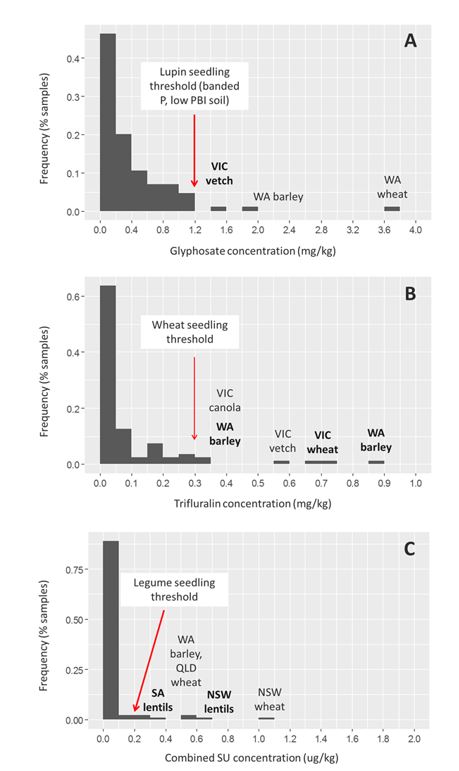
Figure 2. Hazard assessment for A) glyphosate, B) trifluralin and C) sulfonylurea residues in soil. Text in bold indicates potential negative impacts on growth of sensitive crop. Normal text indicates paddocks exceeded thresholds for sensitive crops but when planted with a tolerant crop unlikely to suffer impacts.
Future research
A newly established project in the GRDC Northern Region will focus on measuring diuron and imazapic residues to minimise potential carryover damage, particularly for grain legumes. This project will develop techniques for determining bioavailable residues of these two residual herbicides and critical thresholds for susceptible crops, which will allow growers and adivsers to weigh up the risk of crop damage prior to planting.
Conclusion
A risk framework was used to guide the determination of impacts of residual herbicides on soil biological functions and potential plant-back issues. Within this framework, assessment of what residues of herbicides were in soil had to be conducted first prior to planting the winter crop. Analysis of 80 paddocks in total, across two seasons identified that trifluralin, 2,4-D, diuron, glyphosate and diflufenican are commonly detected in soils. Interestingly, residue levels between 2015 and 2016 were not substantially different, despite analyses of different paddocks in different regions. This data may provide further guidance for future studies. Importantly, the project has clearly identified the lack of major impacts of herbicides on soil biological functions. When herbicides are used as per label instructions, it is unlikely that they will have any long term or significant impact on soil biology. However, risk assessment studies showed some examples where residual herbicides at planting may impact on crop establishment. This was particularly noted for legumes which tend to be more sensitive and the impacts displayed included lower nodule formation, which impacts biological N2 fixation.
Useful resources
- https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2016/02/herbicide-residues-in-soils-are-they-an-issue-northern
- https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2018/02/impacts-of-residual-herbicides-on-soil-biological-function
- https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2018/08/improving-crop-productivity-on-sandy-soils
References
Van Zwieten, L., Rose, M., Zhang, P., Nguyen, D., Scanlan, C., Rose, T., McGrath, G., Vancov, T., Cavagnaro, T., Seymour, N. and Kimber, S., 2016. Herbicide residues in soils–are they an issue? GRDC Grains Research Update, p.117.
Rose, T.J., Van Zwieten, L., Claassens, A., Scanlan, C. and Rose, M.T., 2018. Phytotoxicity of soilborne glyphosate residues is influenced by the method of phosphorus fertiliser application. Plant and Soil, 422(1-2), pp.455-465.
Mick Rose, Lukas Van Zwieten, Pei Zhang, Duy Nguyen, Craig Scanlan, Terry Rose, Gavan McGrath, Tony Vancov, Timothy Cavagnaro, Nikki Seymour, Stephen Kimber, Abby Jenkins, Anders Claassensand Ivan Kennedy. 2016 Herbicide residues in soil – are they an issue? Adelaide GRDC Grains Research Update.
Rose, M.T., Ng, E.L., Weng, Z.H., Wood, R., Rose, T.J. and Van Zwieten, L., 2018. Minor effects of herbicides on microbial activity in agricultural soils are detected by N-transformation but not enzyme activity assays. European Journal of Soil Biology, 87, pp.72-79.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support. We would also like to thank the National Paddock Survey Project team and the advisers and growers contributing to that project.
Contact details
Mick Rose
mick.rose@nsw.dpi.gov.au
GRDC Project Code: DAN00180,
Was this page helpful?
YOUR FEEDBACK