Earwigs – latest research on these damaging pests
Author: Matthew Binns (CSIRO Black Mountain), Ary Hoffmann (University of Melbourne), Maarten van Helden and Thomas Heddle (SARDI) and Paul Umina (University of Melbourne and cesar Australia) | Date: 22 Aug 2019
Take home messages
- Adult European earwigs are present all year, but eggs and juvenile earwigs are only present from April to November.
- European earwigs can cause significant damage to canola, lucerne, lupins and in some cases, lentils.
- The potential for damage to emerging crop seedlings by adult European earwigs exists, although it is dependent upon the timing of germination and egg laying. The juvenile earwigs can cause further damage as the crop develops through winter.
- Multiple earwig species have been detected in grain crops, most of which are not associated with crop damage. It is therefore important to correctly identify which species is present in a paddock before taking any action.
Background
There are at least 80 species of earwigs (Dermaptera) present in Australia (Haas 2018). Recent work using a combination of morphological and molecular data has identified ten different species of earwigs in grain crops (Stuart et al. 2019). These species are comprised of introduced and native earwigs, of which there is not a lot known. However, some of the potentially beneficial species appear to be region specific (Stuart et al. 2019). Reports of earwig outbreaks and damage to canola, cereals and pulse crops, have been increasingly recorded in Victoria, NSW, SA, and southern WA. However, the factors that influence the risk of earwig outbreaks and crop damage are not well documented.
The most dominant and widespread species in Australian crops is the European earwig (Forficula auricularia) (Hill et al. 2019). The European earwig consumes a variety of foods (plants, invertebrates, fungus, detritus), acting beneficially through the consumption of crop pests in orchards (Quarrell et al. 2017). However, the European earwig has been observed as an irregular pest of grain crops in Australia (Murray et al. 2013). Despite being introduced into Australia over a century ago (Quarrell et al. 2018), there has been little research conducted on crop damage from European earwigs in Australia. Furthermore, the potential beneficial activities of the European earwig in grain crops have not been explored. The two dominant native earwig species found in grain crops are Labidura truncata and Nala lividipes (unpublished data). Research has shown L. truncata to be predatory (Horne and Edward 1995), although this species’ importance as a beneficial in grain crops has not been explored. N. lividipes is a pest of Queensland cereals (Hargreaves 1970), although it is unclear if this is also the case in southern Australia or if it will feed on canola at all. It is suspected that increased stubble retention and the associated increase in organic matter within the soil may be linked to greater in-field earwig populations. However, resources documenting the risk of crop damage, as well as management options to minimise the crop damage from pest earwigs, are limited.
Methods
Lifecycle monitoring of the European earwig
To establish the life cycle of European earwigs within a grains production context, grain crops (canola, wheat, oats) at five commercial farms were examined; two in Victoria and three in SA. Sampling at these sites was undertaken over a period of seven days each month from September 2016 to December 2018. During each sampling period two types of traps were used; shelter traps in the form of cardboard rolls inserted into PVC pipe, and pitfall traps containing 100% propylene glycol. Three pitfall traps were installed two meters apart at four sampling locations (>30m apart) per site, with at least one sampling location being placed at the paddock edge for a total of 12 pitfall traps. Pitfall traps were used to capture earwigs during times of activity (i.e. foraging for food at night).
At each of the four sampling locations within the field, three cardboard rolls were placed complementary to the pitfalls to determine abundance (12 rolls in total). The cardboard roll traps were placed within an inter-row parallel to the stubble row, two metres from the associated pitfall and collected seven days later. Additionally, to establish movement of earwigs into vegetation adjacent to field sites, two rolls were placed in the canopy of four trees per site (eight tree rolls) using wire to tie the rolls to branches. The use of cardboard rolls allowed the collection of live earwigs during times of inactivity (being nocturnal they use the rolls for shelter during the day).
European earwigs (F. auricularia) comprised 75% of earwigs collected (unpublished data), and so laboratory processing first involved separating them from the other earwig species that were captured. Part of the species separation involved confirmation via molecular work with comparisons to published sequence data (Stuart et al. 2019). The adult and nymphal stages of F. auricularia was identified using head width, the number of antennal segments and wing bud development as described in Crumb et al. (1941). As earwigs are dimorphic, sex was identified using the shape of the cerci (Crumb et al. 1941) and categorised into male, female and gynandromorph.
Damage and management of the European earwig in canola
The following methods are part of a larger ongoing field experiment, they give background to the data collected and presented in the results but do not cover the entire experiment.
On the 23 April 2019, 30 plots (8m by 1.8m in size) of canola were sown into wheat stubble on a 0.5ha paddock on the Ginninderra research station near Canberra ACT. The plots were randomly allocated seed with different seed treatments; fipronil, imidacloprid or untreated. All seed was planted at the rate of 2.7kg/ha, and all seed was coated with Jockey® Stayer® for blackleg control.
On the 23 May 2019, seedling establishment was measured in the fipronil/imidacloprid/untreated plots by counting the number of seedlings. At this time the European earwigs had either laid eggs or were preparing to lay, but no juveniles had hatched. By mid-June, second instar earwig nymph activity was detected using a modified wildlife camera and significant damage was seen over the next couple of weeks. On the 24 June 2019 the damage to the plots of canola was assessed by estimating biomass loss in each experimental plot.
Results and discussion
Lifecycle
Over the course of two years, 25 000 European earwigs (F. auricularia) were captured from five sites. This provided enough data to understand the lifecycle of the European earwig in grain crops in southern Australia.
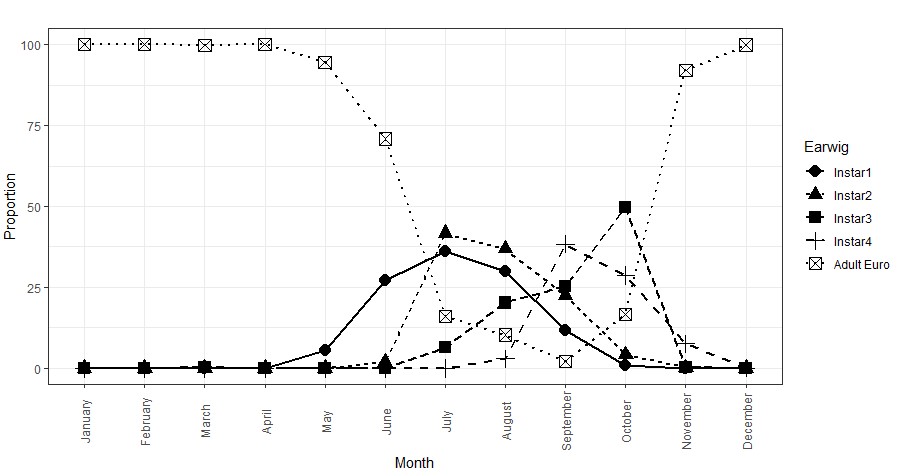
Figure 1. The lifecycle of the European earwig in grain crops. This figure shows the combined proportion (%) data of each F. auricularia lifestage from two years of sampling at five sites across Victoria and SA. Adults are present all year, but juveniles are only present from April to November.
The timing of the peak abundance of each lifestage was relatively consistent across each of the five sites, for which the data was combined to determine the proportion of each lifestage expected to be observed for each month of the year (Figure 1). The initial brood of eggs was observed in nests during April-July, with peak numbers of first and second instar nymphs observed in July. Third instar numbers peaked in October, while fourth instar numbers peaked before this in September. This is potentially due to earwigs moving out of the paddocks and into nearby trees when they reached fourth instar in September and October (Figure 2 - note the log scale abundance axis). Adult earwig numbers were shown to peak in November/December and remain abundant until the following March.
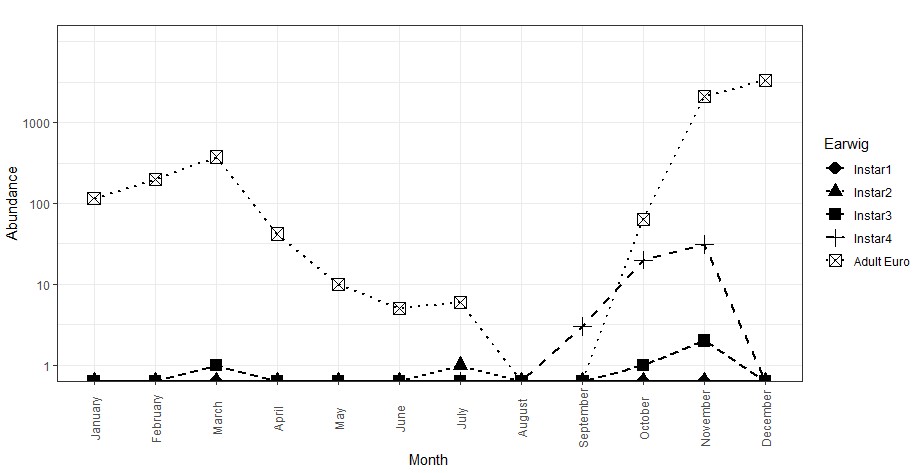
Figure 2. Mean abundance of European earwigs found in trees over time, note log scale axis. Adult numbers decline in the trees during the breeding phase from April to September where they burrow into the ground and care for developing juveniles. Fourth instar juveniles and freshly moulted adults generally move out of the paddock and into the trees over summer.
The collection of detailed lifecycle data for F. auricularia has enabled orchard growers worldwide to preserve or increase earwig numbers and thereby take advantage of their beneficial behaviors to protect orchards from pest insects (Nicholas et al. 2005; Quarrell 2013). Conversely, using this lifecycle data for grain crops may allow better prediction of the risk of crop seedlings to earwig attack, and if necessary, optimise timing of management tactics to reduce earwig numbers by targeting the appropriate lifestages.
Damage and management
The results for this section are part of an ongoing experiment. The results presented are based on incomplete preliminary data but suggest some initial patterns.
Seed coating
No significant difference was found in seedling establishment a month after sowing between seed treatments (p=0.25). Generally, canola is sown and starts growing several weeks before the European earwigs eggs hatch. As such, the severe damage caused by juveniles may be avoided during the plant’s most vulnerable stage depending on when sowing occurs. However, adults will be present at this time and have the capacity to cause seedling damage (cesar, unpublished data), though there is a window of a few weeks where the adult female is underground caring for her eggs and not feeding. For this particular trial, the canola germinated as the females stopped feeding and started laying eggs. Thus, only very mild and occasional earwig damage was seen, not enough to significantly affect seedling establishment. Of course, there are many cases when earwigs can be damaging in autumn months. Although preliminary, these trials point to a complexity of factors at play that influence timing of events and thus impact the likely damage caused to emerging crop seedlings by European earwigs.
Once the earwig juveniles had hatched and reached second instar, significant damage started to occur to the canola (Figure 3). The damage caused by earwig nests at the 4 to 5 true leaf stage was significantly different (p<0.05) depending on the seed coating used (Figure 4). Fipronil provided significantly more protection over untreated and imidacloprid treated seed, while imidacloprid seemed to result in no significant improvement over untreated seed in terms of earwig damage. In laboratory studies, both imidacloprid and fipronil prevented earwig damage during the time in which the coating remained active (cesar, unpublished data). However, fipronil caused significant mortality in earwigs whereas imidacloprid did not (this chemical only acted as a feeding repellant). Potentially, fipronil is more effective in the long term due to the initial reduction in earwig numbers. Thus, as seen in this field trial, damage occurred after the imidacloprid seed coating had worn off and there were significantly fewer (p<0.05) earwig nests found in the fipronil treated areas.

Figure 3. Damage to canola by second instar earwig juveniles. This is typical for canola that is in very close proximity to a European earwig nest, the white arrow (left) points to where the nest was found. The black arrow (right) points to an image of second instar earwigs feeding captured from about 11:00pm to 2:00am.
In this experiment, the overall average biomass loss per plot was 0.17% for fipronil and about 1% for untreated/imidacloprid. This is with an average of eight ‘nesting spots’ for untreated/imidacloprid and 1.6 for fipronil; each ‘nesting spot’ represents either one earwig nesting location or several in very close proximity. The damage does not seem to spread much further than 30cm from a single earwig nest during the pre-bolting growth stage of canola. This demonstrates that while the damage can look bad in isolation, it won’t necessarily result in enough damage to significantly impact overall biomass or yield. This result is from one paddock only, and so additional data is required to assess how this changes with increased earwig nest densities, and how dense they need to be for losses to occur.
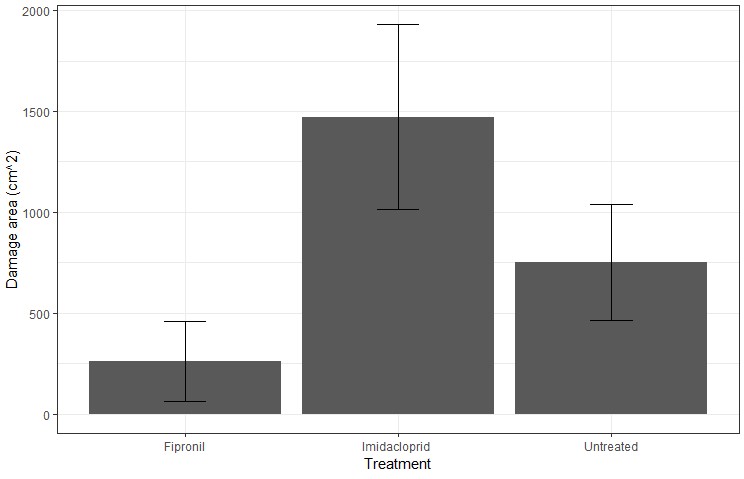
Figure 4. Total area of earwig damage measured in each 8m x 1.8m plot at the 4-5 true leaf canola growth stage. Bars represent the means with +- standard error. Letters represent similar means at p<0.05.
Native Earwigs
The common native species found in grain crops are Labidura truncata and Nala lividipes. Laboratory trials are underway at CSIRO, Black Mountain to determine the roles these species are likely to play in various crop environments. So far, pilot studies have indicated that neither L. truncata nor N. lividipes will feed on untreated canola seedlings under any of the conditions tested so far (including aphid/canola choice and canola only no-choice). L. truncata ate large numbers of green peach aphids when provided, hunting during both day and nighttime. N. lividipes ate smaller numbers of aphids than L. truncata and also cannibalised each other (pers. obs.).
Conclusion
European earwigs are generally not regarded as a pest in grain crops internationally (other than occasional harvest contamination), and potentially play a beneficial role in pest management (Sunderland and Vickerman 1980). However, it is clear from damage reports that they can cause significant damage in Australian crops, particularly canola. European earwigs have the capacity to cause damage throughout the entire season. The nesting phase begins around April, which is often when canola is sown. Prior to nest establishment, adult earwigs are actively feeding, and can cause damage to emerging seedlings if the crop starts germinating at that time. However, there is a period of several weeks where the female earwig is confined underground caring for her eggs and crop feeding is less likely to happen. After the eggs have hatched and the juveniles start developing, significant damage to canola can occur which can be particularly harmful if the plant is still small and vulnerable. Hence, the timing of earwig feeding activity and crop vulnerability is likely to be an important predictor of crop damage.
European earwigs only have one generation per year (with potentially two broods per female), and they don’t travel very far to feed as young juveniles. Once a nest is established, it is likely that the canola near the nest will be damaged, but the earwigs within that nest are likely to cause minimal damage to other parts of the crop. Extensive damage will be the result of many nests becoming established across large areas of the paddock. Monitoring for nests from April until June will allow for management before the earwigs reach the more destructive second instar stage from late June.
The accurate identification of what species of earwig is in the crop is the most important predictor of crop damage. Of the three dominant species found in canola, only European earwigs are confirmed as causing damage, while N. lividipes and L. truncata appear to have beneficial value. The pest activity of N. lividipes in winter cereals will be investigated although it may be season specific, as it is primarily reported as a pest of Queensland sorghum and maize during summer (Hargreaves 1970).
Our data so far suggests that the damage per earwig nest is spatially limited, but very high numbers distributed across a paddock will need to be controlled. So far, the data obtained around control methods is limited. The preliminary results of our trial suggest that fipronil might be useful as a seed coating in areas with a history of earwig problems. However, there is no registration for this pest. Due to the beneficial aspects of earwigs, reducing populations unnecessarily may have consequences for future crops. Therefore, it is important to properly identify which species of earwig is in the field before taking action.
Useful resources
Insect pests of establishing canola GRDC Publication
References
Crumb S. E., Eide P. M. & Bonn A. E. (1941) The European earwig. US Department of Agriculture.
Haas F. (2018) Biodiversity of Dermaptera. Insect Biodiversity: Science and Society.
Hargreaves J. R. (1970) The black field earwig. Queensland Agricultural Journal 96 , 391–392.
Hill M. P., Binns M., Umina P. A., Hoffmann A. A. & Macfadyen S. (2019) Climate, human influence and the distribution limits of the invasive European earwig, Forficula auricularia, in Australia. Pest Management Science 75 , 134–143.
Horne P. A. & Edward C. L. (1995) Phenology and Food Preferences of Labidura truncata Kirby (Dermaptera: Labiduridae) in Western Victoria. Australian Journal of Entomology 34 , 101–104.
Murray D. A., Clarke M. B. & Ronning D. A. (2013) Estimating invertebrate pest losses in six major Australian grain crops. Australian Journal of Entomology 52 , 227–241.
Nicholas A. H., Spooner-Hart R. N. & Vickers R. A. (2005) Abundance and natural control of the woolly aphid Eriosoma lanigerum in an Australian apple orchard IPM program. BioControl 50 , 271–291.
Quarrell S. R. (2013) The chemical ecology, genetics and impact of the European earwig in apple and cherry orchards. PhD Thesis. University of Tasmania.
Quarrell S. R., Arabi J., Suwalski A., Veuille M., Wirth T. & Allen G. R. (2018) The invasion biology of the invasive earwig, Forficula auricularia in Australasian ecosystems. Biological Invasions 20 , 1553–1565.
Quarrell S. R., Corkrey R. & Allen G. R. (2017) Predictive thresholds for forecasting the compatibility of Forficula auricularia and Aphelinus mali as biological control agents against woolly apple aphid in apple orchards. BioControl 62 , 243–256.
Stuart O. P., Binns M., Umina P. A. et al. (2019) Morphological and Molecular Analysis of Australian Earwigs (Dermaptera) Points to Unique Species and Regional Endemism in the Anisolabididae Family. Insects 10 , 72.
Sunderland K. D. & Vickerman G. P. (1980) Aphid feeding by some polyphagous predators in relation to aphid density in cereal fields. Journal of Applied Ecology.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support. This research is part of a national GRDC project (CSE00059) being led by CSIRO and including colleagues at SARDI, NSW DPI, cesar and the University of Melbourne. Thanks to Michael Nash for earwig collection in SA. Thanks to Sarina MacFadyen, Jo Holloway, Hazel Parry and Dusty Severtson for input on experimental design and project management. Thanks to Isobel Roberts for general assistance in the field and lab.
Contact details
Matthew Binns
CSIRO Ag & Food
GPO Box 1700
Canberra ACT 2601
0262464860
Matthew.Binns@csiro.au
GRDC Project Code: CSP1805-016RTX,
Was this page helpful?
YOUR FEEDBACK
