Pre-emergent herbicide: a review of what we know and need to know
Pre-emergent herbicide: a review of what we know and need to know
Author: Roger Mandel, Adjunct Senior Lecturer Curtin University; BASF Australia, Senior Technical Specialist | Date: 04 Feb 2020
Key messages
- Several new pre-emergent herbicides for the control of annual ryegrass are being introduced for use in broadacre cropping.
- Each of the new molecules will have their own advantages and disadvantages.
- This review outlines what we need to know about the physical chemistry of pre-emergent herbicides to get the best efficacy out of them, while rotating them within a cropping cycle.
Abstract
Farmers and agronomists make pre-emergent herbicide decisions based on numerous factors including compatibility with their sowing program, control spectrum and price. Larger farm programs are potentially forced into dry sowing without knock-downs, which puts added pressure on pre-emergent herbicides. Incorporation by sowing (IBS) has inherent risks as it involves placing a chemical capable of killing germinating weeds within centimeters of sown crop seeds. Selectivity of control for most pre-emergent herbicides is based on physical separation rather than crop tolerance and this makes understanding the physical-chemistry properties of pre-emergent herbicides very important. To make informed herbicide decisions we need information but more importantly we need to understand what that information means. As herbicide systems become more complex, our understanding of how they interact with soil, water and biological materials becomes more important. The aim of this paper and presentation is to provide a better understanding of how to interpret the physical-chemistry properties of pre-emergent herbicides to maximise their control.
How dry is dry soil?
The water content of soil even under the harshest summer conditions is still about five per cent of the total soil volume as the water forms a thin film tightly bound to soil particles. The higher the clay content, the higher the amount of water that is held. This water is unavailable to plants but can form solution and sustain some microbial activity. As soil dries, solutes dissolved in the bound water continue to concentrate until some becomes insoluble and crystalises. This small amount of water allows pre-emergent herbicides to bind and reach an equilibrium between the bound and free state. As this water is unavailable for plant uptake, seeds germinating at depth are not likely to be affected by the herbicide.
Volatility
Volatility measures the ability of a substance to transition to the gas state from a solid (sublimate) or liquid (evaporate). The level of volatility determines how quickly a herbicide needs to be incorporated before it is lost to the atmosphere. There is a lot of confusion surrounding volatility with pre-emergent herbicides such as trifluralin. Hollingsworth (1980) showed that volatility loss was low in dry soil (lack of sublimation) but increases when the soil wets up. This indicates that the product is bound in dry soil, but the general industry belief is that trifluralin has an advantage in dry conditions because of the vapour spreading through the soil. Again, this goes to show how understanding physical-chemistry is complex and can led to misinterpretation.
Solubility
Solubility remains one of the most misinterpreted properties of herbicide physical-chemistry. Most people believe it refers to how easily the product dissolves. In fact, it is defined as the maximum concentration of a product in a volume of water. Some chemicals readily go into solution but have low solubility while some chemicals are difficult to dissolve but once dissolved have a high solubility. Solubility is most often confused with soil water partition coefficient (Kd and Koc). As a result, decisions on which pre-emergent herbicide or mix to use under different soil moisture conditions are often made based on the wrong physical-chemistry property.
Koc
Koc is often used by agronomists as an indication of how readily a herbicide will bind to or wash off stubble. This is an important consideration with the high stubble loads of an IBS system. Again, there is a common misunderstanding of how to interpret these physical-chemistry parameters.
First definitions of soil adsorption coefficients or Kd:
![]()
Kd is measured by partitioning using a model soil, it is a lab-defined property of a chemical. The fraction of chemical bound to soil particles and unbound in the soil water film sets up an equilibrium. If herbicide is removed by any means (uptake, detoxification by microbes or leaching), more will be released from the bound state. If the water content of the soil drops, more herbicide will bind. The time required to establish an equilibrium varies between chemicals but can take upward of a couple days. What most people think of as solubility is really Kd or the readiness of a chemical to go into solution.
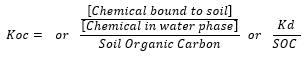
Koc, on the other hand, is a calculated value based on Kd and a standard soil organic carbon (SOC) of ~1%. Globally, agricultural soil ranges from 0.5 to 2.4% SOC and Koc is reported over this range. However, Koc in isolation has little meaning. Herbicides can have a high unit activity, meaning that a very little amount of unbound herbicide can still elicit a high level of performance. Chemicals with a high Kd or Koc will have more chemical bound to the soil and therefore be less prone to leaching and will also have less chemical available for weed uptake and microbial degradation. These and numerous other considerations are made during a product’s development work.
Washing off plant material
The degree to which pre-emergent chemicals wash off plant material depends on whether the plant is dead or alive. Most, but not all, pre-emergent herbicides need to kill the weed before the weed reaches the soil surface and starts photosynthesising. Lipid biosynthesis inhibitors (Groups J, K and one Luximax Group Z) attempt to starve the plant of the building blocks for membranes which are essential for meristem activity and cell elongation. Groups D and E disrupt mitosis or microtubule formation which are also essential for cell division at the meristem. Green plant material will take up pre-emergent herbicides but because of their mode of action the herbicides will not be lethal. Herbicides hitting stubble or dead weeds vary in their ability to bind but this is not directly related to the Koc.
DT50
This chemical property is an indication of the herbicide’s degradation rate or half-life (the number of days that pass until only half of the product is left or still active). Some people think that having half of a product left gives a reduced rate of control but DT50 is determined on the molecule present not on the activity. As with Koc, the majority of product is bound to the soil and not available for weed control but is also unavailable for breakdown. So DT50 and Koc need to be considered together to fully understand a product’s longevity. In product development and registration short DT50s can be overcome by increasing the rate of product, therefore the value is somewhat meaningless unless numerous other pieces of data are also presented.
Root vs. shoot uptake
Pre-emergent herbicides are often mistakenly thought to be preferentially taken up by weed roots or shoots (the coleoptile of monocots, or the epicotyl of dicots). However, I argue that the part of the weed that takes up the herbicide depends more on where the herbicide is located. If a pre-emergent herbicide has low mobility (high Koc and low solubility) or is poorly incorporated (disk sowing system) then the plant tissue most likely to meet the herbicide is the coleoptile or epicotyl (above the seed in dicots). A more mobile herbicide will move into a larger volume of soil and will encounter more plant tissue (both roots and shoots). Non-mobile chemicals require better application techniques to produce a uniform barrier through which the coleoptile must pass through. Clumps and vegetation, both living and dead, cause breaks in the barrier and allow escapes. Root uptake is more effective if the herbicide’s mobility level does not result in leaching loss beyond the root zone. Newly germinating seeds produce only a few branching roots but a very large number of tiny root hairs (root hairs are not small roots but outgrowths of the epidermal tissue to increase water uptake). Seeds do not need any external substances other than water to successfully get their first true leaves to the surface and begin photosynthesising. Secondary roots do not develop until a source of energy, photosynthesis, has been established. Most pre-emergent herbicides must act before the seedling reaches the light so proper positioning of the herbicide is essential for maximum efficacy. Herbicides that are soluble in the weed-seed zone are more likely to be taken up in the bulk flow of water than herbicides in the zone above the seed.
Conclusion
Physical chemistry properties are only useful if we understand both how they are generated and how they interact with each other. Any one property is meaningless in isolation and can potentially lead to poor decisions if the property is misinterpreted. The best way of using a herbicide is as recommended on the registered product label. Changing how a herbicide is used (rates, application method and timing etc.) almost always compromises efficacy as this influences how much chemical is available for uptake, how it is bound to the soil and where in the soil profile it is located. Use patterns that compromise the efficacy of a herbicide not only reduce weed control, but also deliver sublethal does of herbicides, which starts the process of metabolic herbicide resistance.
References
Hollingsworth E B, Volatility of Trifluralin from Field Soil, Weed Science V.28, Mar. 1980, pp. 224-228
Contact details
Roger Mandel (PhD) BASF Australia Ltd.
0429 948 027
roger.mandel@basf.com
