Update on herbicide resistance status in the Western Australian wheatbelt
Update on herbicide resistance status in the Western Australian wheatbelt
Author: Mechelle Owen, Hugh Beckie | Date: 15 Apr 2020
Key messages
- High levels of resistance to Group A and B herbicides for ryegrass and wild radish
- Resistance in wild oat, brome grass and barley grass are low for most commonly used herbicides
- Resistance levels vary across and within cropping regions for different species and herbicides
Aims
To monitor the frequency and distribution of herbicide resistance in key weed species in WA cropping paddocks.
Introduction
Herbicide resistance is a major threat to the long-term sustainability of grain production through the loss of crop productivity and the economic costs of weed control. In Australia, broadacre cropping programs invest heavily in continuous cropping and no-till systems that rely on herbicides for weed control (Llewellynet al 2012). Consequently, the evolution of herbicide resistant weed populations are now widespread in Australia for major crop weeds, including Lolium rigidum (annual ryegrass), Raphanus raphanistrum (wild radish), Avena spp. (wild oat), Bromus spp. (brome grass) and Hordeum spp. (barley grass) (Brosteret al 2011; Boutsaliset al 2012; Owenet al 2014, 2015a,b; Owen and Powles 2016). The GRDC-funded random surveys conducted in Australia reveal that herbicide resistant weeds species are common. However, the incidence of resistance varies significantly for weed species in cropping regions both within a cropping zone and across Australia.
Method
Seed collection
Seed material was collected during October and November 2015 as part of a broadscale survey evaluating herbicide resistance in key weed species. Farmers provided farm maps that were used to locate properties at the time of seed collection. The whole western region was surveyed for target weed species including annual ryegrass, wild oat, wild radish, brome grass and barley grass. During the collection, emerging weed species including Arctotheca calendula (capeweed) and Emex australis (double gee) were also collected. In total, 509 crop fields were visited just before grain harvest. Crop fields were chosen at random and weed seed collected by two people walking in an inverted ‘W” pattern across each field. During sampling, weed density ratings were recorded and mature seedheads were collected from many plants (50-100 plants, bulked at collection). After collection, seed heads were rubbed and chaff removed by aspiration (wild radish seed pods were milled to release the seeds and chaff material was then separated by aspiration). Seed samples were stored in a warm, dry glasshouse with a daily average temperature of 300C over the summer months (December to April) to relieve any seed dormancy.
During March 2018, farmers were contacted and provided Conyza spp. (fleabane) samples for herbicide resistance screening. At the same time, roadsides were sampled in the southern agricultural region of WA, by stopping at 5km intervals where fleabane was present. Samples were collected from flowering plants and bulked from the sample site.
Seed germination
Wild radish seeds were sown directly into trays of moist potting mix in the autumn and winter of 2016. Annual ryegrass was germinated on agar at 20°C for five days and then transplanted into trays of potting mix during May to October 2016/17. In the 2017/18 growing season, brome grass and barley grass seeds were pre-germinated on agar for 10-14 days at 4°C, then transplanted into potting mix. During the 2018/19 growing season, wild oat seeds were scarified by nicking to relieve physical dormancy and germinated using the same techniques as brome and barley grass. Wild oat seedlings were transplanted into potting mix. For screening with pre-emergent herbicides, seedlings were transplanted after three days, when the radicle was just visible. Capeweed and double gee seeds were placed on the soil surface and covered with 2cm of soil. Fleabane seedlings were germinated on agar for seven days and then transplanted into trays containing potting mix. All species were grown to the appropriate growth stage before herbicides were applied.
Herbicide resistance screening
When seedlings had reached the appropriate growth stage, they were sprayed with the highest recommended field rate of the herbicides commonly used for the control of each species using a dedicated cabinet sprayer and protocols optimised in previous surveys (Owen et al 2007, 2014). Seedlings were then grown outdoors for another 21 days with regular watering and fertilisation. Depending on seed numbers, each population was screened once or twice, with 50 seedlings per treatment. Plant mortality was assessed 21 days after treatment by determining whether the growing point was chlorotic or new growth was visible, and by comparing with well-characterised susceptible and resistant (where available) control populations. Populations were classified based on the number of individual plants surviving each herbicide treatment. Susceptible populations were classified as those having 0% plant survival. Resistant populations were classified into two groups: those having 1 - 19% plant survival (hereafter denoted as <20%) and those having ≥20%. To clarify, these categories of lower and higher resistance define the proportion of plants surviving herbicide application, not the level of resistance of an individual plant. Populations in the lower category can still contain highly resistant individuals capable of surviving higher than the recommended rates and going on to produce (resistant) seed. For fleabane, 100 seedlings of each population were treated with glyphosate and 50 seedlings were treated with 2,4-D.
Results
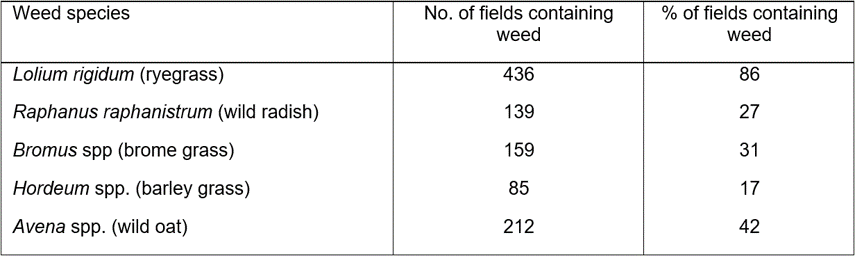
A total of 509 cropping paddocks were visited just before harvest in the Western Australian wheatbelt. A total of 348 annual ryegrass, 65 wild radish, 128 wild oat, 97 brome grass, 42 barley grass, 47 capeweed, five double gee, and one silver grass populations were collected from crop fields (Table 1). In 2018, 94 fleabane populations were collected from farmer fields and roadside locations.
Table 1. Number and percentage of fields containing each weed species
Annual ryegrass
During 2016, annual ryegrass populations were treated with several herbicides to determine their resistance spectrum. Of the 338 populations treated with the ACCase-inhibiting herbicide diclofop, 96% of populations contained resistant plants while 83% of populations also had resistance to sethoxydim. Only 44% of populations had resistance to clethodim at the higher rate (Table 2). For the ALS-inhibiting herbicides, 99% of populations had sulfometuron-resistant plants, while 97% contained plants resistant to imazamox + imazapyr. Only 2% of populations had plants resistant to atrazine (photosystem II inhibitor). The knockdown herbicides glyphosate (EPSPS inhibitor) and paraquat (photosystem I inhibitor) provided good control of most ryegrass populations. No populations had resistance to paraquat, while some populations displayed resistance to glyphosate (Table 2). Resistance to the pre-emergence herbicide trifluralin (microtubule disruptor) showed a small increase, with 30% of populations showing some level of resistance (Table 2). Resistance was detected to the newer pre-emergence herbicide Boxer Gold® (prosulfocarb+S-metolachlor: lipid synthesis inhibitor) and to Sakura® (pyroxasulfone).
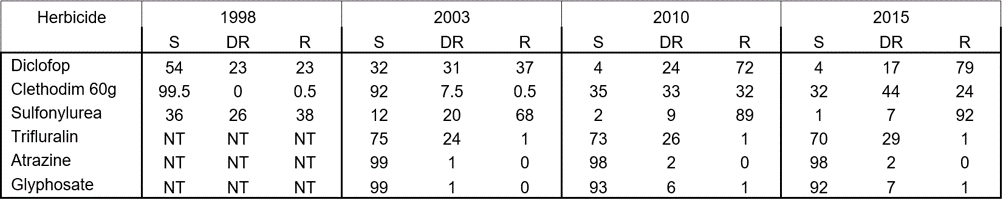
Resistance in ryegrass has not increased dramatically since 2010 for commonly used herbicides (Table 3), however there have been small changes in resistance frequencies. Although there was only a small increase in the number of glyphosate-resistant populations, resistance is no longer confined to the higher rainfall zones on the south coast of WA (Owen et al 2014).
Table 2. The percentage of annual ryegrass populations with resistance to each herbicide from the 2015 survey collection
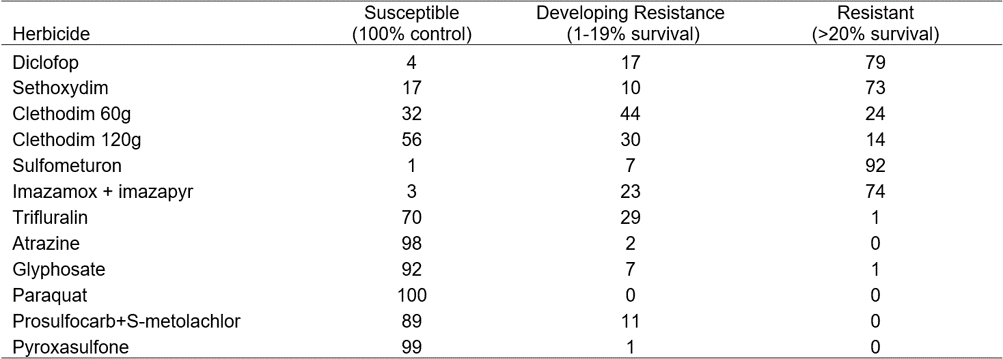
Table 3. Changes in the percentage of ryegrass resistance for each herbicide and category between 1998 and 2015. S (susceptible – 100% control), DR (developing resistance 1-19% survival) and R (resistant > 20% survival), NT (not tested).
Wild radish
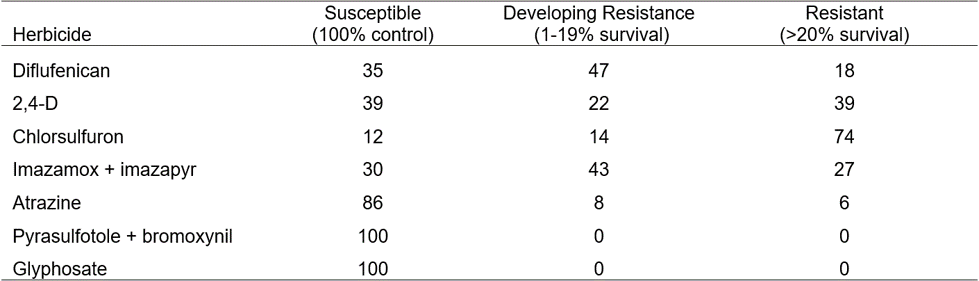
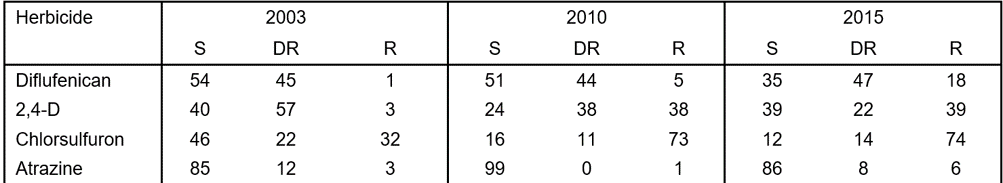
During the 2016 growing season, collected wild radish populations were treated with a range of herbicides. Of the 65 populations sprayed with the ALS-inhibiting herbicide chlorsulfuron, 88% of populations had resistant plants. Also, 70% of populations had cross resistance to the ALS herbicide mixture imazamox + imazapyr (Table 4). For the synthetic auxin 2,4-D, 61% of populations contained resistant plants, while no populations exhibited resistance to the knockdown herbicide glyphosate. Screening with atrazine and diflufenican (PDS inhibitor) indicates there are some populations displaying resistance to these herbicides. Resistance levels have varied over the past 12 years (Table 5).
Table 4. The percentage of wild radish populations with resistance in each category from the 2015 survey collection
Wild radish is most prolific in the northern agricultural region. There were fewer wild radish populations collected in 2015 from the northern agricultural region compared to past surveys. Wild radish numbers in other regions of the wheatbelt were similar to previous years.
Table 5. Changes in the percentage of wild radish resistance for each herbicide and category between 2003 and 2015. S (susceptible – 100% control), DR (developing resistance 1-19% survival) and R (resistant > 20% survival).
Brome and barley grass
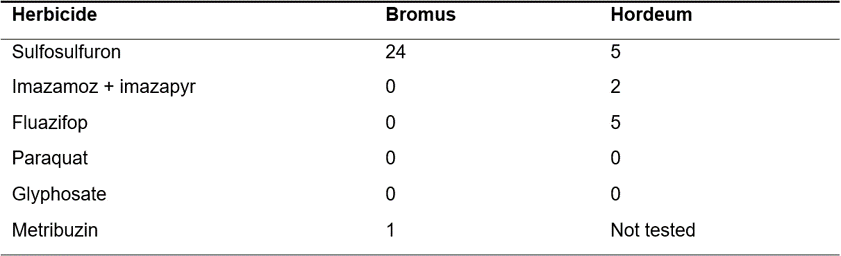
During the 2017/18 growing season, brome and barley grass seedlings were treated with herbicides to determine their resistance profile. Of the 96 brome grass and 42 barley grass populations, there was no resistance to clethodim, glyphosate or paraquat, however two barley grass populations displayed resistance to fluazifop. Some brome and barley grass populations also had plants resistant to the ALS-inhibiting sulfonylurea herbicide sulfosulfuron (Table 6). Resistance levels in barley grass populations were consistent with the previous survey in 2010, however SU resistance in brome grass has increased from 12 to 24% during the same time period.One population of brome grass was resistant to metribuzin (photosystem II inhibitor).
Table 6. Percentage of tested Bromus and Hordeum populations resistant to each herbicide group from 2015
Wild oat
During the 2018 growing season, wild oat seedlings were treated with herbicides to determine their resistance profile. Of the 128 wild oat populations screened to the ACCase-inhibiting herbicides, 52% had plants resistant to diclofop while 18% had plants resistant to fenoxaprop, 10% to tralkoxydim and 5% to pinoxaden. For the ALS inhibiting herbicide, 6% of populations had some level of resistance to mesosulfuron, while no resistance to the imidazoline herbicides was detected. For the late season herbicide flamprop, 28% of populations had resistant plants. These resistance levels in wild oat are similar to that of the 2010 survey, although there has been a small increase in resistance for the ALS SU herbicides from 2% to 6% and for flamprop from 8% to 24%. No populations have shown resistance to glyphosate or paraquat, however there is some evidence of triallate resistance in a small number of populations and this will be evaluated further during 2020.
Fleabane
During the 2018 growing season (April/May), fleabane populations were treated with glyphosate and 2,4-D. These treatments were repeated in September and any plants surviving the glyphosate treatment were grown on for seed. The progeny from these populations were treated with several doses of glyphosate during September 2019 to determine their resistance status. Of the 94 fleabane populations, 11 populations were confirmed resistant to glyphosate, while no populations displayed resistance to 2,4-D.
Capeweed
From April to June 2019, 47 capeweed populations were treated with glyphosate, sprayseed ® (diquat + paraquat) and Lontrel ® clopyralid. All treated populations were susceptible to clopyralid and glyphosate, however, 10% of these populations had plants resistant to sprayseed. Populations will be screened with 2,4-D in the 2020 growing season.
Conclusion
Herbicide resistance is widespread in annual ryegrass populations in most agricultural fields for commonly used herbicides although resistance levels vary across regions and between species. Other grass weeds, namely wild oat, brome grass and barley grass are not as widespread as annual ryegrass and tend to be associated with rainfall regions or agronomic factors. Despite these species being less abundant, resistance to the ACCase- and ALS-inhibiting herbicides commonly used to control these species was identified. It is important to document the distribution and herbicide resistance frequency in these minor species to establish baseline data for continued surveillance and to raise farmer awareness to help mitigate adverse economic impacts. Continual monitoring of the distribution, incidence and frequency of herbicide resistance will aid management decisions by farmers and agronomists for successful, sustainable weed control using a wide variety of cultural, mechanical and chemical options, including weed seed management techniques at harvest that prevent weed seeds from replenishing the seed bank. The challenge is to use a wide range of integrated weed management options to help achieve herbicide sustainability and productivity of cropping systems.
Acknowledgments
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and GRDC investment and the authors would like to thank them for their continued support. We also thank the growers and consultants and Paula Reeve, John Quealy, Roslyn Owen-Rechichi and Danica Goggin, who provided invaluable technical assistance in many areas of the research that contributed to this paper.
References
BoutsalisP, Broster J, Koetz E, Wu H (2012) Herbicide resistance frequencies in ryegrass (Lolium spp.) and other grass species in Tasmania. Plant Protection Quarterly 27, 36-42.
Broster J, Koetz E, Wu H (2011) Herbicide resistance levels in annual ryegrass (Lolium rigidum Gaud.) in southern New South Wales. Plant Protection Quarterly 26, 22-28.
Llewellyn RS, D'Emden FH, Kuehne G (2012) Extensive use of no-tillage in grain growing regions of Australia. Field Crops Research 132, 204-212.
Owen MJ, Walsh MJ, Llewellyn RS and Powles SB (2007) Widespread occurrence of multiple herbicide resistance in Western Australian annual ryegrass (Lolium rigidum) populations. Australian Journal of Agricultural Research, 58, 711-718.
Owen MJ, Martinez NJ, Powles SB (2014) Multiple herbicide-resistant Lolium rigidum (annual ryegrass) now dominates across the Western Australian grain belt. Weed Research 54, 314-324.
Owen MJ, Martinez NJ and Powles SB (2015a) Multiple herbicide resistant wild radish (Raphanus raphanistrum) populations dominate Western Australian cropping fields. Crop and Pasture Science 66, 1079–1085.
Owen MJ, Martinez NJ and Powles SB (2015b) Herbicide resistance in Bromus and Hordeum spp. in the Western Australian grain belt. Crop and Pasture Science 66, 466-473.
Owen, MJ and Powles SB (2016) The frequency of herbicide resistant wild oat (Avena spp.) populations remains stable in Western Australian cropping. Crop and Pasture Science 67, 520–527.
‘’® Registered trademark’’
Contact details
Mechelle Owen
Senior Research Officer
Australian Herbicide Resistance Initiative
University of Western Australia
35 Stirling Hwy, Crawley, WA
Ph: 0417984266
Email: Mechelle.owen@uwa.edu.au
