Exploring interactions of arbuscular mycorrhizal fungi (AMF), rhizobia and root-lesion nematode (Pratylenchus thornei) in mung bean
Exploring interactions of arbuscular mycorrhizal fungi (AMF), rhizobia and root-lesion nematode (Pratylenchus thornei) in mung bean
Author: Elaine Gough, Kirsty Owen, Rebecca Zwart, Alla Marchuk and John Thompson (Centre for Crop Health, University of Southern Queensland) | Date: 18 Aug 2020
Take home message
- Arbuscular mycorrhizal fungi (AMF) and rhizobia acted together to increase nodulation, plant biomass and seed yield in mung bean cv. Jade-AU. This synergism led to an increase in uptake of nutrients, including N, P and K in the plant tops resulting from the mycorrhizal symbiosis and increased rhizobial nodulation
- Macronutrients and micronutrients essential to the nodulation process such as calcium, copper, magnesium, boron and manganese increased in the dual-inoculated plants
- Rotations with mycorrhizal associated crops to increase the levels of AMF in the soil followed by soil tests using PREDICTA®B for AMF could be beneficial prior to planting mung bean to ensure adequate nodulation by rhizobia
- Following a long fallow or non-host crop, soils are low in AMF. Build up populations of AMF using low mycorrhizal dependency crops such as wheat and oats before moving to higher mycorrhizal dependency crops like mung bean
- P. thornei population densities were higher on mung bean cv Jade-AU when inoculated with P. thornei and AMF, compared to P. thornei inoculated plants alone. This increase was only on inoculation with high population densities of 10 P. thornei/g soil and was not related to increases in root biomass by AMF inoculation at this high rate. Further research is required as interactions may differ dependent on host genotype, cultivar and AMF species.
Background
Mung bean is an important summer pulse crop, with an estimated farm gate value of approximately $100 million each year. The advantages of integrating mung bean into cropping systems include its ability to fix nitrogen (N) and the added benefit as a water efficient, short season high value crop. The variety Jade-AU has a market share of approximately 60% of mung bean produced in the region (Col Douglas pers comm). Economic reduction and loss of yield in mung bean in the northern grain region can be attributed to bacterial and fungal pathogens causing diseases such as tan spot, halo blight, powdery mildew and other constraints such as nodulationfailure (Murray & Brennan, 2012).
Arbuscular mycorrhizal fungi (AMF), previously known as vesicular arbuscular mycorrhizal fungi (VAM) are beneficial organisms found associated with the roots of 80% of terrestrial land plants including many agriculturally important crop species. They form a symbiotic relationship, exchanging the plants photosynthetic carbon for improved uptake of water and nutrients such as phosphorus (P) and zinc (Zn) from the soil (Parniske, 2008). They may also play a role as bio-protectants against fungal, bacterial, and nematode pathogens (Yang et al., 2014). There are many species of AMF fungi, which for diagnostic purposes are categorised into seven molecularly distinguished groups. Group A, which includes the species Funneliformis mosseae and group B, which includes the species Claroideoglomus etunicatum are commonly found in the northern grain region. Spore levels of AMF in the soil are higher following mycorrhizal crops than fallow or non-mycorrhizal crops like canola. Some crops have a higher mycorrhizal dependency for growth such as sorghum, maize, sunflower, faba bean, linseed, chickpea and mung bean than others such as barley and millet. Crops grown in soils with low levels of available phosphate are dependent on AMF for nutrient acquisition (Smith & Read, 2010) and for highly dependent crops extremely high rates of P fertiliser are required to offset lack of AMF.
Rhizobia are bacteria that form a symbiosis with legumes to fix N gas from the air into ammonium. The role of rhizobia in N fixation is well known and inoculation of legumes with rhizobia is currently practised in agriculture worldwide. Mung bean has the capacity to meet the N requirements for growth and yield through biological N2 fixation (Gentry, 2010). However, previous surveys undertaken in the northern grain region indicate that the majority of mung bean crops are poorly nodulated and fix only 30% of their requirements for N (Herridge et al., 2005). Nodulation failure is a constraint to mung bean crop production that can lead to yield reductions of up to 50% (Gentry, 2010).
Nodulation failure can be ascribed to various reasons, such as incorrect inoculum application, unsuitable soil pH, high levels of soil nitrate, and deficiencies of nutrients such as phosphorus and molybdenum (Drew et al., 2012). Limitations of certain nutrients including phosphorus, calcium, boron and magnesium can affect the development and functionality of nodules and therefore productivity of the leguminous crop (O’Hara, 2001). Synergism between rhizobia and AMF can increase mineral nutrition and plant growth, nodulation levels and N fixation in other legumes (Javaid, 2017). Our hypothesis is that one cause of nodulation failure of mung bean in the northern grain region could be a lack of AMF propagules in the soil.
Also found in the soils of the northern grain region are root-lesion nematodes, Pratylenchus spp., which feed in and migrate through root tissue, negatively affecting root function and can lead to yield loss in intolerant host crops (Owen et al., 2019). Pratylenchusthornei followed by P. neglectus are the most important species of Pratylenchus found in the northern region (Thompson et al., 2008). Murray and Brennan (2009) estimated that P. thornei can causeAUD $104 million in potential losses annually to wheat in the sub-tropical grain region.Mung bean is a susceptible host to P. thornei and any increase in nematode populations can have detrimental effects on yields of subsequent intolerant crops.
Both Pratylenchus spp. and AMF occupy a similar ecological niche in the root cortex of the host plant. The coexistence of AMF and plant-parasitic nematodes in roots and soil has prompted a number of investigations into their interactive effects on plants. These investigations document the generally suppressive effect that AMF have on migratory nematodes (Yang et al., 2014), although some studies showed no effect or even an increase in nematode numbers after dual-inoculation with AMF (Pinochet et al., 1996; Hol & Cook, 2005). These conflicting results may be due to differences in environmental and nutritional factors, AMF species, nematode species and/or crop hosts (Gough et al., 2020).
Nematodes can also affect nodulation levels which may result in a decrease in functionality in chickpea (Castillo et al., 2008). Most research on the interaction between rhizobia, nematodes and AMF has been with root-knot nematodes Meloidogyne spp. and there is little information on how Pratylenchus spp. affect the levels or efficiency of nodulation. Hussey and Barker (1976) found Pratylenchus penetrans stimulated nodule formation on soybean but inhibited N fixing capacity. Synergism between AMF and rhizobia has been demonstrated in other leguminous crops such as chickpea, soybean and French bean as reviewed by Chalk et al., (2006).
The interaction between host, parasitic nematode, and beneficial symbionts is likely to be quite specific. So far, there has been no research into these interactions for mung bean in the vertosols of the northern grain region.
Methods
A glasshouse experiment with mung bean and factorial treatments of AMF, rhizobia and P. thornei in a randomised split plot design was conducted in 2018. The P. thornei susceptible mung bean cv. Jade-AU was sown in pots containing steam pasteurized Vertosol from the Darling Downs with no additional fertiliser. The experimental soil properties were as follows; pH 8.5 (1:5 water), nitrate- N 24.5 mg/kg, Colwell phosphorus 45 mg/kg, zinc 1.45 mg/kg (DTPA).
The soil temperature was maintained at ~22°C and the air temperature was maintained at ~25°C. Pots were watered with a capillary bottom watering matting system under 8 cm water tension. Inoculations at planting included three rates of P. thornei (0, 1 or 10 nematodes/g soil (oven dried (O.D) equivalent) and two rates of an isolate of AMF species Funneliformis mosseae, from the northern grains region (0 or 16 spores/g soil O.D equivalent). The Bradyrhizobium used was the commercially available CB1015 strain. Plants were assessed at 6 and 12 weeks after planting. Plant biomass, seed pod count, seed weight, nodule counts and root biomass, P. thornei population density, AMF % colonisation of the roots, and leaf chemical concentrations and uptakes were determined. Data were analysed using ANOVA followed by Fishers protected l.s.d using Genstat. Data for nodulation was square root transformed and P. thornei numbers were log transformedprior to statistical analysis.The experiment was repeated in part of a larger factorial experiment looking into interactions of AMF, P. thornei, rhizobia and nutrients in 2019 using 10 P. thornei/g O.D soil equivalent soil and 16 spores F. mosseae/g soil O.D soil equivalent.
Results
AMF and rhizobia interactions increased nodulation, biomass, seed yield and nutrient uptake in mung bean
Inoculation with both AMF and rhizobia resulted in a synergistic effect (Figure 1) which significantly (P<0.001) increased nodule numbers, dry biomass, seed weight, plant uptake of nitrogen, phosphorus, potassium and zinc in the mung bean plants (Figure 2, Figure 3 a-b, Figure 4). The uptake of other macronutrients and micronutrients associated with rhizobia nodulation increased in the dual inoculation; macronutrients: calcium (P<0.001), magnesium (P<0.01): micronutrients: boron (P<0.001), copper (P<0.001) and manganese (P<0.01).

Figure 1. The addition of AMF and rhizobia to mung bean increased plant biomass and seed yield four-fold at 12 weeks after inoculation in Experiment 1.
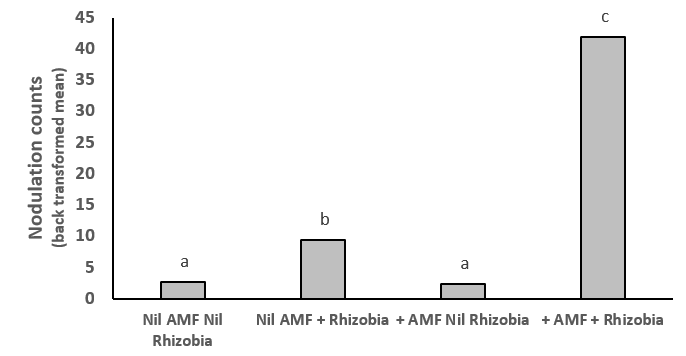
Figure 2. AMF and rhizobia increase nodulation 4.5-fold compared to rhizobia alone in mung bean. Different letters indicate significant differences at P=0.05.
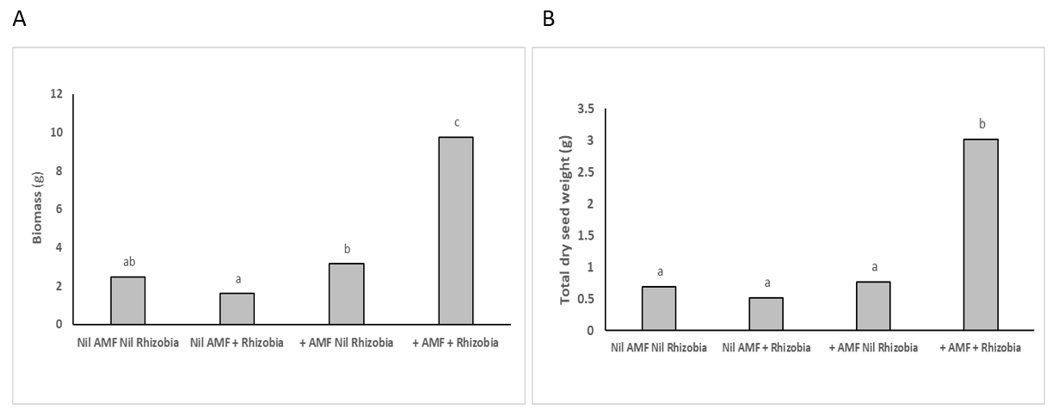
Figure 3. Synergistic effects of inoculation with both AMF and rhizobia at 12 weeks in Experiment 1 resulted in 4-fold increase in biomass (A-left) and seed weight (B-right) in mung bean. Different letters indicate significant differences at P=0.05
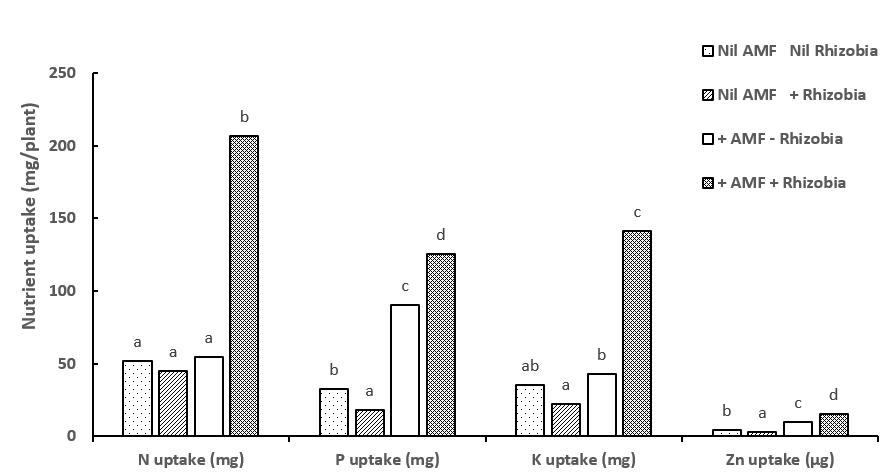
Figure 4. Synergistic effects of inoculation with both AMF and rhizobia at 12 weeks in Experiment 1, resulted in increased N uptake (mg/plant); P uptake (mg/plant); K uptake (mg/plant) and Zn uptake (µg/plant) in mung bean. Different letters indicate significant differences at P=0.05.
AMF and P. thornei interactions in mung bean
Plants inoculated with AMF increased the reproduction rate of P. thornei in the plant at high initial inoculum rates of 10 P. thornei/g O.D soil equivalent (P< 0.05) (Figure 5), but not at lower rates of 1 P. thornei/g O.D soil equivalent. The increase in reproduction at higher rates was not as a result of increased plant above-ground biomass or root biomass (Figure 6 b). However, increases in reproduction at lower rates of 1 P.thornei/g was correlated with increased plant root dry weight (Fig 6 b).
Inoculation with P. thornei did not affect nodulation rates of rhizobia, plant biomass or seed yield of mung bean.
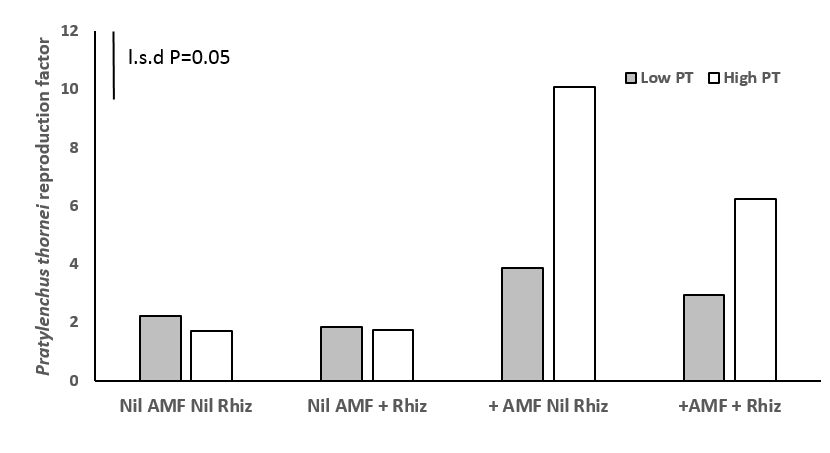
Figure 5. AMF increased reproduction of Pratylenchus thornei (PT) at higher rates of 10 P. thornei/g soil in mung bean after 12 weeks growth in Experiment 1.
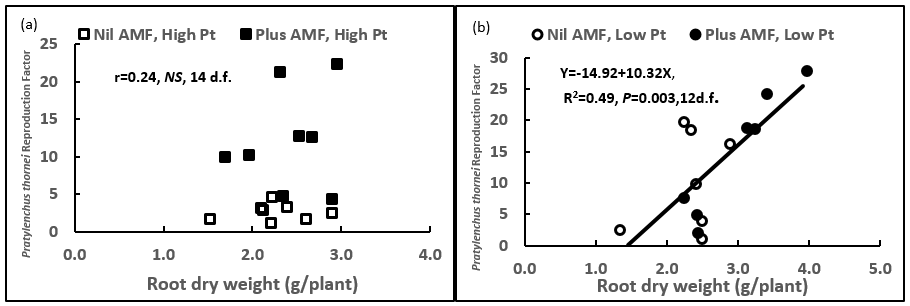
Figure 6. The higher reproduction factor (RF) of P. thornei was not caused by an increase in plant root biomass in mung bean at high initial rates of 10 P.thornei/g (Fig. 6 a), but was correlated at low rates of 1/g P. thornei (Fig 6 b).
Conclusions
Inoculating mung bean cv. Jade-AU with both AMF and rhizobia led to a large increase in the number of nodules, plant biomass and seed yield compared to rhizobia alone. This dual inoculation increased the uptake of nutrients N, P and K in the plant. It also increased the uptake of other nutrients essential to the metabolism of rhizobia and the establishment of an effective symbiosis such as Ca, Mg, Mn, Cu and B.
Establishing adequate levels of AMF in the soil, a soil analysis of AMF by PREDICTA®B prior to planting mung bean, and inoculation with the correct isolate of rhizobia, may lead to increased nodulation thereby increasing yield productivity of mung bean.
Management practices to establish adequate AMF levels can be achieved through rotations with crops that associate with mycorrhizae. As AMF require a living host to survive, soils after a long fallow or cultivation of a non-host crop such as canola, can reduce AMF propagules in the soil. Growing crops that have a lower mycorrhizal dependency such as wheat and oats, after a long fallow may increase the AMF propagules in the soil for a subsequent crop that has a higher mycorrhizal dependency (Thompson et al., 1997). Growing mung bean after mycorrhizal crops that are also poor hosts of P. thornei (Thompson 1994) would also be a good strategy.
Mungbean cv Jade-AU plants inoculated with both AMF and P. thornei had an increase in the reproduction rate of P. thornei as compared to plants inoculated with P. thornei alone at high initial populations of 10 P. thornei/g soil. This was not related to increases in root or above-ground biomass.
However, variability in Pratylenchus population densities in the presence of AMF may be genotype dependent and may also be influenced by the species of AMF. AMF can increase the tolerance of plants to infestation by plant-parasitic nematodes (Schouteden et al, 2015). The increase in nutrient uptake from improved AMF colonisation, resulting in a more vigorous plant, may be a strategy to counteract the reduction in root quality and root efficiency conferring a compensatory effect against the damage done by the nematode. More research is needed on the interactions of AMF, rhizobia and Pratylenchus spp. in other mung bean genotypes and other host crops.
Rotations of crops to increase AMF spore densities and reduce P. thornei population densities are desirable in cropping systems in the northern grain region.
References
Castillo, P, Navas-Cortés, JA, Landa, BB, Jiménez-Díaz, RM & Vovlas, N (2008) Plant-parasitic nematodes attacking chickpea and their in planta interactions with rhizobia and phytopathogenic fungi, Plant Disease, vol. 92, no. 6, pp. 840-53.
Chalk, P, Souza, RdF, Urquiaga, S, Alves, B & Boddey, R (2006) The role of arbuscular mycorrhiza in legume symbiotic performance, Soil Biology and Biochemistry, vol. 38, no. 9, pp. 2944-51.
Drew, E, Herridge, D, Ballard, R, O’Hara, G, Deaker, R, Denton, M, Yates, R, Gemell, G, Hartley, E & Phillips, L (2012) Inoculating legumes: a practical guide,GRDC
https://grdc.com.au/__data/assets/pdf_file/0023/400865/GRDC_Inoculation-of-Legumes2019LoRes.pdf
Gentry, J (2010) Mungbean management guide. DEEDI
Gough EC, Owen KJ, Zwart RS, Thompson JP (2020) A review of the interactions between arbuscular mycorrhizal fungi and the root-lesion nematode Pratylenchus. Frontiers in Plant Science. doi: 10.3389/fpls.2020.00923
Herridge, D, Robertson, M, Cocks, B, Peoples, M, Holland, J & Heuke, L (2005) Low nodulation and nitrogen fixation of mungbean reduce biomass and grain yields, Australian Journal of Experimental Agriculture, vol. 45, no. 3, pp. 269-77.
Hol, WHG & Cook, R (2005) An overview of arbuscular mycorrhizal fungi–nematode interactions, Basic and Applied Ecology, vol. 6, no. 6, pp. 489-503.
Hussey, RS & Barker, KR (1976) Influence of nematodes and light sources on growth and nodulation of soybean, Journal of Nematology, vol. 8, no. 1, pp. 48-52.
Javaid, A (2017) Role of AMF in nitrogen fixation in legumes, in A Zaidi, et al. (eds), Microbes for Legume Improvement, Springer International Publishing, Cham, ch 17, pp. 409-26.
Murray, GM & Brennan, JP (2009) Estimating disease losses to the Australian wheat industry,Australasian Plant Pathology, vol. 38, no. 6, pp. 558-70.
Murray, GM & Brennan, JP (2012) The current and potential costs from diseases of pulse crops in Australia: GRDC Research Code: CER00002. Barton, ACT: GRDC.
O'Hara GW (2001) Nutritional constraints on root nodule bacteria affecting symbiotic nitrogen fixation: a review. Australian Journal of Experimental Agriculture, vol 41, pp 417-433.
Owen K, Simpfendorfer S, Thompson J, Sigel L (2019) Management strategies to help reduce losses against root lesion nematodes. Northern region Root lesion nematode Fact Sheet, GRDC May 2019.
Parniske, M (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses, Nature Reviews Microbiology, vol. 6, no. 10, pp. 763-75.
Pinochet, J, Calvet, C, Camprubí, A & Fernández, C (1996) Interactions between migratory endoparasitic nematodes and arbuscular mycorrhizal fungi in perennial crops: A review,Plant and Soil, vol. 185, no. 2, pp. 183-90.
Schouteden, N, De Waele, D, Panis, B, and Vos, CM (2015) Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: A review of the mechanisms involved. Frontiers in Microbiology, vol 6, pp 1280.
Smith, SE and Read, DJ (2010) Mycorrhizal symbiosis. Academic press.
Thompson, J (1994) Concurrent management of symbiotic VAM fungi and pathogenic root-lesion nematodes through crop rotation. In ‘Soil biota–management in sustainable farming systems’.(Eds CE Pankhurst, BM Doube, VVSR Gupta, PR Grace) pp. 119–120, CSIRO Publishing: Melbourne.
Thompson, JP, Bowman, R, Seymour, NP, Peck, D and Clewett, TG (1997) VAM boosts crop yields. The natural way to healthy crops. QDPI Farming Systems Institute Crop Link Research Focus8 pp.
Thompson, JP, Owen, KJ, Stirling, GR & Bell, MJ (2008) Root-lesion nematodes (Pratylenchus thornei and P. neglectus): a review of recent progress in managing a significant pest of grain crops in northern Australia.
Yang, H, Dai, Y, Wang, X, Zhang, Q, Zhu, L & Bian, X (2014) Meta-analysis of interactions between arbuscular mycorrhizal fungi and biotic stressors of plants, The Scientific World Journal, vol. 2014, pp. 7.
Yeates, G, Ross, D, Bridger, BA & Visser, TA (1977) Influence of the nematodes Heterodera trifolii and Meloidogyne hapla on nitrogen fixation by white clover under glasshouse conditions, New Zealand Journal of Agricultural Research, vol. 20, no. 3, pp. 401-13.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support.
The PhD research is supported by a USQ research scholarship and a GRDC Research Scholarship to Elaine Gough. A special thanks to the Crop Nematology team at the Centre for Crop Health, USQ for support of this project and to QDAF for access to glasshouses at Leslie Research Facility.
Contact details
Elaine Gough
Centre for Crop Health
University of Southern Queensland
Email: elaine.gough@usq.edu.au
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
GRDC Project Code: USQ1912-003RSX,
