2020 - the year of the armyworm
2020 - the year of the armyworm
Author: Jessica Lye, Julia Severi (Cesar Australia), Paul A. Umina, Garry McDonald (Cesar Australia and University of Melbourne), Rebecca Hamdorf, Maarten van Helden, Kym Perry (SARDI) | Date: 24 Feb 2021
Take home messages
- The incidence of some pests varies dramatically from year to year; the reports of large infestations of native species of armyworms were very prominent in 2020.
- Drought breaking rains, mild winter temperatures and even changing cropping practices may have contributed to the outbreak of armyworms. The recent arrival of fall armyworm in Australia will further complicate this.
- PestFacts services in south eastern Australia have a 14 year history of assisting the grains industry with identification services, managing invertebrate pests, and with recording pest incidence across time.
- As with other pests, correct identification of armyworms, is an important first step in sustainable management.
Background
‘Identification, surveillance and advisory platform for management of grains pests’ (CES1904-002RTX) is delivered by cesar Australia, DPIRD, QDAF, NSW DPI, and SARDI. It aims to provide grain growers and advisers with timely in-season information on invertebrate grain pest occurrence and to equip industry with the knowledge needed to implement integrated pest management practices. One delivery platform for this project is PestFacts south-eastern.
PestFacts south-eastern keeps growers and advisers informed about invertebrate pests and beneficials in broadacre crops and pastures during the winter-cropping season in Victoria (Vic) and southern New South Wales (NSW). It has been running since 2006 and provides regular updates to a subscribership of over 1300 grains advisers and growers throughout southern NSW and Vic. Equivalent platforms are the PestFax service (grains western region), PestFacts South Australia, and the Beatsheet (Grains northern region).
PestFacts south-eastern can demonstrate a long history of tracking and collating field observations. Pest reports, and more recently, reports of beneficial species, have been logged in the PestFacts Map database since 2006. This database includes uploaded reports from both PestFacts South Australia (coordinated by the South Australian Research and Development Institute) and from PestFacts south-eastern (coordinated by cesar Australia). Therefore, the database includes over a decade of field reports from across south eastern Australia.
Not all reports are verified (for instance, some reports are verbal or provided over email and lack a digital image or sample for identification). Therefore, this database provides an indication rather than an absolute picture of pest and beneficial occurrences over a given season. In addition, there are potential influencing factors that may bias reports, which should be taken into account when using information from this database.
For instance, reporting is likely to be influenced by changes in agronomist knowledge over time (e.g., an improved understanding of how to differentiate pest species) and raised awareness about certain pests (e.g., public coverage of exotic pest incursions). Nevertheless, when assessed in context, this dataset remains a powerful tool for retrospectively considering pest occurrences over time.
Armyworm species in south eastern Australia
‘Armyworm’ is a catch-all common name for the larvae (caterpillars) of several related moth species spanning temperate, sub-tropical and tropical climates. The types of armyworm that most grain growers and advisers are most familiar with in south eastern Australia are those that prefer cereals and grasses. These include three native species: the common armyworm (Mythimna convecta), the southern armyworm (Persectania ewingii), and the inland armyworm (Persectania dyscrita).
These native species as larvae are difficult to tell apart by eye; reliable identification requires adult moth specimens. Nevertheless, identifying each individual species in the field is generally not critical because their broad habits, type of damage and control are similar.

Figure 1. The three parallel light stripes on an apparent ‘collar’ behind their head (prothoracic shield) is a key feature of the common armyworm, southern armyworm and inland armyworm. The light stripes often, but not always, persist along the abdomen. (Image credit: Julia Severi, cesar Australia).
In February 2020, the fall armyworm ‘FAW’, Spodoptera frugiperda, was first reported on the Australian mainland in far north Queensland (Qld). The pest has rapidly spread and has now been detected across parts of Qld, Northern Territory (NT), NSW, and Western Australia (WA).
Models developed by cesar Australia (Plant Health Australia, 2020) and Du Plessis et al. (2018) show that FAW will likely be observed in a wide range of growing regions, but the expected seasonal activity will vary depending on climatic conditions. While northern parts of Australia will support permanent, year-round populations, migrations into southern regions, such as central WA, Victoria’s high rainfall region or the Mallee region in SA and Vic are predicted to occur from spring as the weather warms.
In this paper, we present an overview of all field reports collected from across south eastern Australia in 2020, with a particular focus on incidences of native species of armyworm throughout this time period.
Results and discussion
Overview of invertebrate reports in 2020
Last year the majority of reports across south eastern Australia to PestFacts services were received from May-August. In 2020, reporting was dominated by detection of lepidoptera, followed by various species of aphid. Overall, reports of armyworm were highest throughout 2020, followed by reports of Russian wheat aphid (Table 1).
During planting and early establishment of winter crops, cabbage centre grub and weed web moth were the most commonly reported species. Cutworm, Russian wheat aphid and armyworm (native spp.) were the most common reports from May-August. As the weather warmed during early spring, native budworm become the most commonly reported species.
Table 1. Pest reports supplied to PestFacts south-eastern and PestFacts South Australia from January-December 2020 for major pest categories. For brevity, some reports are omitted (e.g., Beneficial reports are not included). Highest reports for each category are bolded.
TOTAL | JAN-FEB | MAR-APR | MAY-JUN | JUL-AUG | SEP-OCT | NOV DEC | ||
|---|---|---|---|---|---|---|---|---|
Aphids | Blue green aphid | 3 | 1 | 2 | ||||
Cabbage aphid | 2 | 1 | 1 | |||||
Cowpea aphid | 25 | 2 | 11 | 6 | 6 | |||
Green peach aphid | 7 | 2 | 4 | 1 | ||||
Oat aphid | 2 | 2 | ||||||
Root aphid | 3 | 3 | ||||||
Russian wheat aphid | 55 | 2 | 32 | 19 | 2 | |||
Turnip aphid | 4 | 4 | ||||||
Mites | Balaustium mite | 2 | 1 | 1 | ||||
Blue oat mite | 9 | 2 | 5 | 1 | 1 | |||
Bryobia mite | 6 | 2 | 3 | 1 | ||||
Redlegged earth mite | 16 | 5 | 11 | |||||
Caterpillars (Lepidoptera) | Beet armyworm | 3 | 3 | |||||
Bronzed field beetle | 5 | 3 | 2 | |||||
Brown pasture looper | 19 | 4 | 15 | |||||
Cabbage centre grub | 12 | 10 | 1 | 1 | ||||
Cutworm | 37 | 8 | 18 | 11 | ||||
Corn earworm | 5 | 3 | 1 | 1 | ||||
Diamondback moth | 3 | 2 | 1 | |||||
Flea beetle | 3 | 2 | 1 | |||||
Grass anthelid | 1 | 1 | ||||||
Grass blue butterfly | 1 | 1 | ||||||
Herringbone caterpillar | 13 | 4 | 7 | 2 | ||||
Armyworm – native spp. | 52 | 2 | 9 | 33 | 8 | |||
Native budworm | 22 | 2 | 3 | 3 | 13 | 1 | ||
Pasture day moth | 5 | 2 | 3 | |||||
Pasture tunnel moth | 4 | 3 | 1 | |||||
Pasture webworm | 8 | 3 | 5 | |||||
Underground grass grub | 1 | 1 | ||||||
Weed web moth | 20 | 1 | 15 | 4 | ||||
Beetles | African black beetle | 6 | 1 | 5 | ||||
Argentine cockchafer | 1 | 1 | ||||||
Black headed cockchafer | 1 | 1 | ||||||
False wire worm | 2 | 2 | ||||||
Little pasture cockchafer | 1 | 1 | ||||||
Vegetable beetle | 1 | 1 | ||||||
Yellow headed pasture cockchafer | 8 | 1 | 7 | |||||
Weevils | Grey banded leaf weevil | 1 | 1 | |||||
Sitona weevil | 1 | 1 | ||||||
Vegetable weevil | 6 | 6 | ||||||
White-fringed weevil | 1 | 1 | ||||||
Others | European earwig | 5 | 4 | 1 | ||||
Leafhopper | 9 | 9 | ||||||
Lucerne flea | 10 | 4 | 5 | 1 | ||||
Millepede | 2 | 2 | ||||||
Rutherglen bug | 11 | 3 | 4 | 4 | ||||
Ryegrass mealybug | 3 | 1 | 2 | |||||
Slaters/Pill bug | 3 | 3 | ||||||
Slugs & snails | 14 | 2 | 11 | 1 |
Early season Lepidoptera activity
Early 2020 represented an unusual situation for weed web moth, which has long been considered a sporadic and minor pest in South eastern Australia, and cabbage centre grub, which is a warm season pest that generally declines rapidly during autumn.
Based on behaviour and morphological features, reports of weed web moth were suspected to be Achyra affinitalis. Reports from the Riverina, South West Slopes, and Central West Slopes and Plains in NSW indicated the presence of high numbers on establishing canola, lucerne and broadleaf weeds. Past records document weed web moth larvae as growing to 15mm. However, during early 2020 several larvae were measured up to 20mm, and even up to 35mm in some instances.
Cabbage centre grub (Hellula sp.) was also found in higher than usual numbers in southern and central NSW, with damage being observed on young canola plants and forage brassica.
Atypical reports
Each year PestFacts south-eastern receives reports of atypical invertebrates in crops. During 2020, the service received a number of unusual reports during the winter cropping season. These were primarily located in the Riverina and Central West Slopes and Plains of NSW. Of note were:
- Flea beetle (Phyllotreta undulata) in canola;
- Rice root aphid (Rhopalosiphum rufiabdominale) in wheat stands; and
- Leafhoppers in canola and other crops.
A high number of armyworm reports
During winter to early summer, reports of armyworm were relatively common in comparison to preceding years. For instance, in 2020 more armyworm reports were received to PestFacts services (PestFacts south-eastern and PestFacts South Australia) than in any year of the preceding 14-year period (Figure 2). In 2020, most armyworm reports were received from the Loddon-Mallee, eastern Wimmera and Eyre Peninsula regions (Figure 3).
According to reporters, significant armyworm damage and large populations were observed in several cases, although there were also many cases where no damage was evident. Reports were also received of armyworm marching behaviour, which happens when populations become stressed and run low on a food source. Examples of reports where feeding damage and high numbers were observed are included below.
Case 1 (Late March):
- Significant damage to an oat crop - quickly moving across one side and leaving no plants behind.
- 2-3 weeks post sowing.
- No real sign of damage one week after sowing.
- Originated from a neighbour's pasture paddock.
- Agronomist monitored the pasture finding 5 - 6 larvae each sweep.
Case 2 (Late July):
- Approximately half a hectare of wheat (GS20-29) observed were plants had no leaf, only stem remaining.
- Larvae were easy to find, feeding on the plants and sheltering under the surface clods of soil.
- There were an estimated >50 larvae per square metre in the most damaged parts of the paddock.
- There was a high amount of stubble residue on the surface.
Case 3 (late August):
- Armyworm were found crawling across a road.
- The caterpillars were dark in appearance.
- They were moving out of a paddock that was ‘eaten out’.
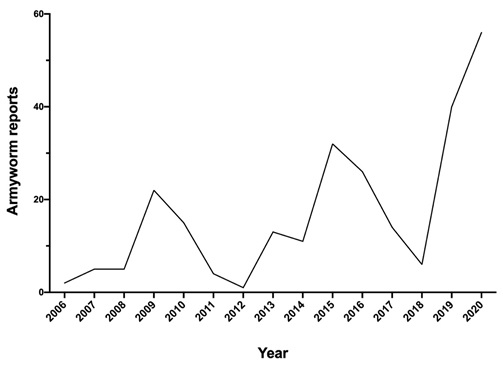
Figure 2. Reports logged as ‘armyworm’ for South eastern Australia over 2006-2020 (Source: PestFacts Map database).
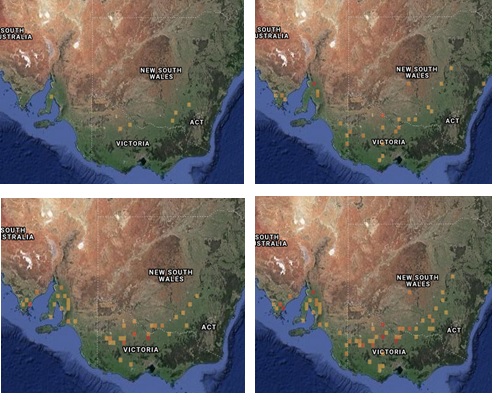
Figure 3. Reports of armyworm to Pest Facts services across three years: 2018 (top left – 6 reports), 2019 (top right – 40 reports), 2020 (bottom left – 55 reports), 2018-2020 (bottom right – 101 reports).
Factors that may influence high armyworm activity and reporting
Many invertebrate pests fall roughly into one of two groups: resident or migrant pests. The latter are inclined to invade crops in seasonal surges. Native species of armyworm are examples of migratory moth species that are well adapted to breed on native and introduced grasses in production and more arid inland regions. When adults of these populations migrate, synoptic conditions that often result in rainfall facilitate their flight, in some instances over hundreds or even a thousand kilometres, to new regions (Farrow and McDonald 1987; Drake and Farrow 1988). Therefore, migrating moths tend to be concentrated in areas where rain is likely. Heavy rains, particularly in autumn, can drive growth of armyworm populations (McDonald 1991). Under the right conditions, armyworm populations can quickly build in numbers as winter grasses and crop host plants enter a vegetative growth phase. Investigation of common armyworm phenology indicates that there are two generations over autumn and winter, with the first generation taking advantage of autumn rainfall, and the second generation being laid in June/July (McDonald et al. 1995).
Outbreak events require adequate populations in source areas (i.e., supported by autumn rainfall in arid inland regions), suitable atmospheric conditions to facilitate moth migration to production regions, as well as rainfall and subsequent vegetative growth of plant hosts within a short time after egg lay. Large outbreaks in Queensland and in parts of NSW following heavy rain events in 1988 and 1989 have been described in McDonald et al. (1995).
In assessing pest bulletins for southern Australia over a period spanning the early 80s, late 80s, early 90s, and 2006-07, Hoffmann et al. (2008) notes a reduced incidence of armyworm outbreaks over this period. The reduction was attributed to dry conditions preventing a build-up of armyworm species – high winter rainfall was rare across the timeframe assessed by Hoffmann et al. (2008). Armyworms are a pest that are well adapted to exploit post-drought conditions. Intense armyworm breeding and slow recovery of natural enemies following the dry weather gives armyworm species a strategic head start.
Such a situation was described in McDonald et al. (1990), which assessed a 1983 armyworm outbreak six months after a nation-wide drought had broken. While some population growth of common armyworm at the time was driven by drought-breaking rains, other large populations were supported by localised rainstorms. Multiple instances of moth emergence from April to November, local and long-range migration of emerged moths, and a substantial increase in suitable habitat was likely to have supported armyworm colonisation of inland NSW during this period.
This post-drought effect is observed for armyworm species found overseas. Janssen (1993) describes in detail the phenology of the African armyworm (Spodoptera exempta) and factors influencing outbreak situations in Tanzania and Kenya. In this situation, outbreaks occur with the first rains of the wet season. They describe a drought-outbreak effect, whereby large numbers of African armyworm are commonly detected after drought-breaking rains.
In 2020, large native armyworm populations were observed at a number of sites across SA, Vic and southern NSW. In line with high reporting of armyworm, above average rainfall was recorded between 1 January to 30 November across NSW and central Vic (where a high number of armyworm reports were made) (BOM, 2020).
Development times and survival of native armyworm are strongly influenced by temperature. Surveys for the common armyworm from 1988-89 found that survival of common armyworm was low over autumn and winter in Vic and high in Northern NSW and Southern Qld (McDonald et al. 1995). The southern armyworm, which has a lower optimum temperature threshold than that of the common armyworm, was found to be more abundant than the common armyworm in more southern latitudes. North of 33° the common armyworm was the dominant species (McDonald et al. 1995).
It is possible that a mild winter in south eastern Australia favoured armyworm species. Across Australia, the January – November 2020 mean temperature was the third warmest on record. This combination of higher than average rainfall, and higher than average temperatures from autumn – spring over much of the country (Figure 4) is likely to have supported the breeding of native species of armyworm in arid inland regions, as well as survival in cropping regions over winter.
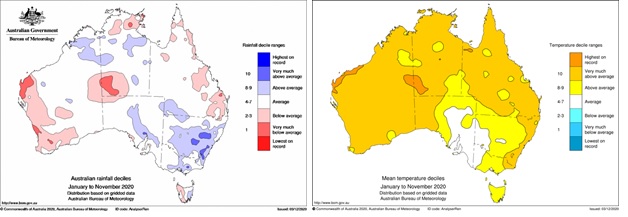
Figure 4. Rainfall deciles for January to November 2020, based on all years 1900 to 2020 (left), and mean temperature deciles for January to November 2020, based on all years 1910 to 2020 (right) (Source: the Bureau of Meteorology)
Another possible contributing factor to the year’s outbreak may have been a gradual shift in cropping practices. For example, Hoffman et al. (2008) suggest that reduced armyworm reports from the early 80s to mid 2000s may have also been a product of changing farming practices. Specifically, rotation with pasture grasses had become less common during this period. Since 2007, armyworm reports have been increasing. Localised outbreaks may be influenced by other practices, such as stubble retention and no-till, with high loads of retained stubble in autumn providing a preferred site for armyworm oviposition.
Several reports in 2020 noted that armyworm had been found in paddocks (often barley paddocks), with a high stubble load. Oviposition behaviour during autumn is central to such a scenario. Armyworm moths lay their egg batches (in their hundreds) in very tight cracks and crevices in gramineae. One known egg-laying site is the remnant crevice between the flag leaf and dead tillers in dried grasses and cereal stubble. Previous observations have demonstrated that green grass pasture (barley grass, Hordeum glaucum) with a component of dried grass had higher densities of common armyworm larvae, in comparison to a pasture lacking the dried grass component (McDonald 1991).
In addition, based on laboratory-based experiments undertaken by McDonald (1991), oviposition options offered by dried grass (cocksfoot – Dactylis glomerata, oats – Ayena sativa, and barley – Hordeum vulgare) are a more highly preferred than those offered by green, actively growing grass of the same species. This preference helps to explain why larger larval populations are often found in stubble-retained paddocks and why cereal crops sown into cereal stubble, particularly standing stubble, can be at a higher risk of armyworm invasion and subsequent feeding damage.
Reporting can be positively influenced by increases in knowledge, awareness campaigns, and prior experience that has led to greater diligence in monitoring. For instance, high numbers of lepidoptera early in the 2020 winter cropping season (e.g., weed web moth and cabbage centre grub) may have primed field staff towards observing lepidoptera throughout the season. In addition, during 2020 the industry become particularly aware of the need to investigate armyworm in paddocks following the northern Australia incursion of FAW in February 2020, which had high media coverage from February – May 2020. Rapid spread of this species to more southern regions has led to many in the grains industry adopting an ‘early detector’ approach.
During the early stages of the FAW incursion, there was notable knowledge accrual behaviour. In early 2020 active learning about armyworm in Australia was evidenced by a large increase in the number of armyworm PestNote page views on the cesar Australia website (2,378 views during 1 February-1 April 2020). The majority of page entrances from February-April 2020 resulted from direct google key word searching. This is in contrast to 2019, when this PestNote was visited 711 times during the same period. Therefore, a higher awareness of armyworm in the field was likely influenced by such preparatory behaviour at the beginning of the winter cropping season.
Additional reports for a particular pest group following an exotic pest incursion is to be expected. This was observed in 2016, with the detection of Russian wheat aphid (Diuraphis noxia) in SA. Figure 5 displays corn aphid (Rhopalosiphum maidis), oat aphid (Rhopalosiphum padi) and Russian wheat aphid reports from 2006-2020. A notable increase in aphid reports occurred in 2016, when Russian wheat aphid was first detected.
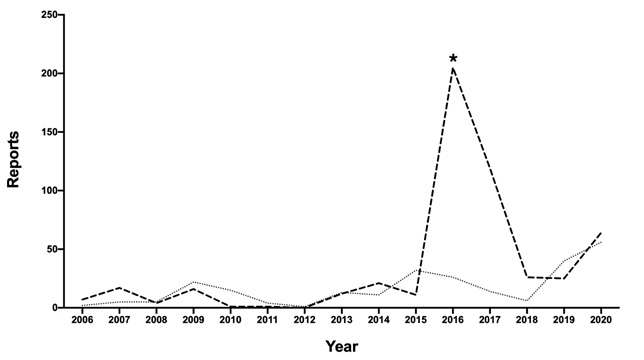
Figure 5. Increase in field pest reports after an exotic species has been detected. Cereal aphid (corn aphid, oat aphid, and Russian wheat aphid) reports from 2006-2020 are shown as the bolded, dashed line. The majority of reports are for Russian wheat aphid. Armyworm reports are included as a dotted line. The asterix indicates when Russian wheat aphid was first detected in Australia.
The importance of distinguishing armyworms
Typical feeding behaviour of common armyworm, southern armyworm and inland armyworm is to feed on leaves and young tiller growth. If the crop is young, this occasionally sets back the crop, but generally there is minimal economic impact unless there’s heavy defoliation. However, as crops dry down, large populations of armyworm can become more voracious as available green material becomes scarce. It is at this point that the larvae that routinely move up the plant, become attracted to the nodes and stems that stay green for longer. If the larvae are a later stage of maturity, head lopping can occur. In Victoria, the common armyworm is most likely to reach a late larval stage when cereals are drying down. In Tasmania, as reported by Hill (2013) it is the southern armyworm that is likely to reach damaging late instar stages when cereals are most susceptible.
Barley and oats are particularly susceptible to damage if mature caterpillars are present in spring, due to their relatively thin stems and the potential for lopping of maturing heads. Therefore, as host crops approach ripening, detecting an armyworm infestation is important in order to make a decision about whether active control is necessary or not.
With the incursion and establishment of FAW in Australia during early 2020, the need to become proficient in field-based armyworm identification is greater. This involves gaining a sound understanding of species phenology as well as morphology, in order to determine when a species of armyworm is likely to be found in the region, and on what hosts.
Based on work on the predicted seasonality of FAW (Du Plessis et al. 2018; Plant Health Australia, 2020), this species is unlikely to be an issue for much of the winter cropping season in southeastern Australia. It requires temperatures to be above a certain threshold for development (approximately 12.5°C for egg to adult development). In addition, it does not have a diapause phase, and therefore, it cannot survive cold winters in an ‘inactive’ state (Luginbill 1928).
However, FAW is highly migratory and capable of dispersing several thousands of kilometres. It is possible that southeastern Australia could receive seasonal migrations of FAW during summer when susceptible crops like maize and sorghum are grown. Ultimately, how Australia’s climatic and vegetation zones will influence the timing and magnitude of migrations is still unknown.
During the warmer months, with much of the industry on alert for the possible spread of FAW into southern cropping regions, it is important to recognise that other species of moth larvae of less importance than fall armyworm can be found in summer field crops.
During the early summer of 2020 one species reported as creating damage in maize and soybeans was the beet armyworm (Spodoptera exigua), also known as the lesser armyworm. This species is thought to be native to south-east Asia, and like several other sub-tropical and temperate countries, it has established in much of mainland Australia. With a very wide host range spanning broadleaf plants and grasses, vegetables, flowering ornamentals (Capinera 2020), maize, cotton, lucerne, sunflower, rice, sorghum, millet, linseed and sesame (Common 1990) it demonstrates host range overlap with FAW.
Field reports in 2020 have demonstrated that beet armyworm larvae can be present in FAW host crops during the warmer months in Vic and southern NSW. However, reports have been infrequent and ‘windowing’ feeding damage is reported as being patchy. Therefore, this species is very unlikely to require chemical intervention. Becoming more familiar with morphological features of the beet armyworm will reduce the chance of a misidentification during monitoring for a FAW incursion.
Like species of native armyworm, which occur in winter cereals, the beet armyworm does not have a hairy appearance and can display some resemblance of the three parallel light stripes on an apparent ‘collar’ behind their prothoracic shield. These lines continue down the length of their bodies, however they are not always present or as distinctive as may be seen on native armyworm species.
The beet armyworm can vary in colour from light green to dark green-brown and have a mottled appearance. It is not uncommon to observe some pinkish pigmentation. They often have a white stripe down their sides, and white spots close to their spiracles. Beet armyworm larvae can be distinguished from Helicoverpa sp. and FAW, by their lack of enlarged spots (pinacula) and lack of coarse hairs.
Aiding entomologists to improve tracking and verification
The level of reporting to PestFacts services has remained relatively stable since 2010, with reporting peaking in 2016-2017 and 2020. A major change in reporting has involved how reports are received, and the format of these reports.
In 2010, reporting was primarily face to face and over the phone, while identification requests were undertaken from live samples. From 2017, with the introduction of the PestFacts Reporter app and increasing usage of Twitter and email, digital images have become the preferred method for requesting an identification (live samples decreased in 2020 in part due to COVID-19 restrictions).
In terms of verifying reports that are uploaded to the PestFacts Map database, the preference towards use of digital samples has been a benefit, with the potential to verify reports having increased from 25% in 2010 to 59% in 2020. Apart from allowing greater opportunity to verify reports, this method reduces cost and time input for the reporter and increases the potential for a faster response from entomologists. However, a greater proportion of digital reports also represents additional challenges. With a greater number of invertebrate identifications to undertake overall, performing identifications efficiently is crucial.
Identification requires the entomologist to clearly view the features of the insect, such as the structures of legs, wings, body hairs, and other appendages, as well as consistency of colour, etc. The ability to verify a report or perform an identification can be hindered by insufficient information and low-quality images. Remembering some basic best practices for taking a high-quality digital image improve the chance of receiving an accurate and timely identification.
Guidelines for supplying a high-quality digital sample
- Optimise focus: Ensure that it is the invertebrate that is most in focus, and not the plant or your hand or the soil.
- Take multiple angles: Identifying an invertebrate from just one angle/photo is not ideal, particularly if they are obscured by soil or plants. Take several photos from various angles such as top down, side view and/or front on, where applicable. Taking photos of several individuals to account for variation in the population is also good practice.
- Use appropriate technology: Carry a 5x or 10x smartphone macro lens that can be clipped over a smartphone camera. They enable a clearer image for smaller invertebrates like aphids and mites, and they can also be used on other types such as caterpillars, moths and beetles. Contact cesar Australia to receive a lens.
- Consider microscopic features: Small features such as hairs, raised spots, segmentation, head form and mouthparts, antennae and legs, all provide key information about the invertebrate. If the subject is relatively large (such as a late instar caterpillar) supply close-up photos of the invertebrate in sections (e.g., thirds).
- Prepare the subject: Very mobile invertebrates, such as mites, can be placed in the fridge for 10-15 minutes prior to taking a photo. This will slow them down.
Additional information that should be sent with the digital sample, where possible, include:
- The location (to the resolution of the locality, e.g. Rochester);
- Host on which the sample was found;
- Growth stage;
- Damage observed;
- Extent of the infestation (e.g. patchy over 1 hectare); and
- Paddock history (previous crop, tilled/not tilled, seed treatments, spray applications).
There will be some circumstances where digital samples are not sufficient for an identification. Therefore, it is good practice to keep a small container in the car so that a live sample can be collected and mailed.

Figure 6. A lateral and dorsal view of a beet armyworm larva (top left and top right) (Image credit: Julia Severi, cesar Australia).
Conclusion
It is important to note that reporting does not necessarily reflect abundance. For instance, reports of lucerne flea were low in 2020, yet this is generally a highly abundant pest. Rather, these pest reports can be used to gain insight into unusual regional issues during the season and to understand what pest species are viewed as significant to the broader industry. Thus, this reporting system is an indirect mechanism for understanding changes to pest pressure and importance (Hoffmann et al. 2008; Mangano et al. 2005).
As a method of determining pest importance, Hoffmann et al. (2008) notes that outbreak bulletins have caveats. Field staff may not correctly identify species, minor outbreaks may not be reported, and these reports do not necessarily reflect the economic importance of a pest outbreak. Reporting can also be influenced by a variety of factors. Increased reporting will occur when:
- A new species is ‘expected’;
- When a species is ‘unusual’ and highly visible;
- When symptoms are very visible and specific (e.g., Russian wheat aphid); and
- When there are issues managing a species in an area (e.g., resistance, no chemical options, an increase due to changes in management).
Reports will decrease in frequency for pests that are ‘common’ and easy to manage. However, in the absence of a structured system of report collection and validation, these reports have potential to be used as a proxy measure.
Hoffmann et al. (2008) had previously assessed southeastern Australian pest reports over a multi-year timeframe in order to identify changes to what pests are deemed as important in the Australian grains industry. It is likely that the importance of pest groups in relation to reporting has once again shifted since that analysis and another assessment of these changes is timely. Through analyses such as the one presented in this paper, and through continued maintenance of databases such as PestFacts Map, the grains industry can more fully understand how pest species cycle in prevalence and importance over many years, and potentially over decades.
Acknowledgment
CES1904-002RTX is delivered by the National Pest Information Network (cesar Australia, DPIRD, QDAF, NSW DPI, and SARDI). This project aims to provide grain growers and advisers with information on invertebrate grain pest occurrence and equip industry with the knowledge needed to implement integrated pest management practices. This initiative is a GRDC investment and includes in-kind contributions from all project partner organisations. The work undertaken is made possible by the significant contributions of growers through the support of the GRDC. PestFacts Map data included in this update was collected and collated by personnel at cesar Australia and the South Australian Research & Development Institute. Acknowledgment goes to all parties who have provided field reports to PestFacts services.
Useful resources
For more information on armyworm, and for threshold advice, visit PestNotes Southern
Watch an example of armyworm marching behaviour on YouTube.
Cesar Australia youtube profile
PestFacts Map can be found on the Cesar Australia and SARDI websites.
Refer to the I-SPY identification manual for information on identifying armyworm species
https://grdc.com.au/resources-and-publications/all-publications/publications/2018/i-spy
References
Bureau of Meteorology (2020) Tracking Australia’s climate through 2020.
Capinera J (2020) Handbook of vegetable pests. Academic Press, Elsevier.
Common I (1990) Moths of Australia. Melbourne University Press, Carlton.
Drake V & Farrow R (1988) The influence of atmospheric structure and motions on insect migration. Annual Review of Entomology. 33: 183-210.
Du Plessis H, van den Berg J, Ota N & Kriticos D (2018) Spodoptera frugiperda – CLIMEX modelling. InSTePP Pest Geography, Canberra
Farrow R & McDonald G (1987) Migration strategies and outbreaks of noctuid pests in Australia. Insect Science and its Application. 8: 531-42.
Hill L (2013) The common armyworm, Mythimna convecta (Walker) (Noctuidae: Lepidoptera), a seasonal resident in Tasmania. Plant Protection Quarterly. 28: 114-119.
Hill L (2014) Lesser armyworm, 'Spodoptera exigua' (Hubner) (Lepidoptera: Noctuidae), a vagrant moth in Tasmania. Plant Protection Quarterly. 29: 31-142.
Hoffmann A, Weeks A, Nash M, Mangano G & Umina P (2008) The changing status of invertebrate pests and the future of pest management in the Australian grains industry. Australian Journal of Experimental Agriculture. 48: 1481–1493.
Janssen J (1993) Soil nutrient availability in a primary outbreak area of the African armyworm, Spodoptera exempta (Lepidoptera: Noctuidae) in relation to drought intensity and outbreak development in Kenya. Bulletin of Entomological Research 83: 579-593.
Luginbill P (1928) The fall army worm. US Dept. of Agriculture.
Mangano P, Bellati J, Henry K, Umina P & Severson D (2005) PestFax and PestFacts - newsletters successfully facilitating interactive communication on invertebrate pest and disease control in broadscale crops and pastures in southern Australia. Extension Farming Systems Journal 5 (2) – Industry Forum
McDonald G, Bryceson K & Farrow R (1990) The development of the 1983 outbreak of the common armyworm, Mythimna convecta, in Eastern Australia. Journal of Applied Ecology. 27: 1001-1019.
McDonald G (1991) Oviposition and larval dispersal of the common armyworm, Mythimna convecta (Walker) (Lepidoptera: Noctuidae). Australian Journal of Ecology. 16: 385–393.
McDonald G, New T, Farrow R (1995) Geographical and temporal distribution of the common armyworm, Mythimna convecta (Lepidoptera: Noctuidae), in eastern Australia: larval habitats and outbreaks. Australian Journal of Zoology. 43: 601–629.
Plant Health Australia (2020) Fall Armyworm Continuity Plan for the Australian Grains Industry, Version 1.
Contact details
Paul Umina
pumina@cesaraustralia.com>
GRDC Project Code: CES1904-002RTX,
