Mechanical soil amelioration alters soil biology, soilborne pathogen and nematode pests of cereal crops: what are the implications?
Mechanical soil amelioration alters soil biology, soilborne pathogen and nematode pests of cereal crops: what are the implications?
Key messages
- The effects of mechanical soil amelioration treatments on soil biology including soilborne pathogen Rhizoctonia solani (AG8) and nematode pests (Pratylenchus neglectus, P. quasitereoides (RLN)and Heterodera avenae (CCN)) populations varied depending on organism, tillage technique and soil type.
- Soil amelioration stimulated soil biological activity and increased populations of R. solani, P. quasitereoides, P. neglectus and H. avenae at 10-40cm depth where they do not usually occur in WA.
- Soil inversion and soil mixing reduced R. solani inoculum in the topsoil, where it commonly impacts crops early in the season. Topsoil R. solani populations remained low over both seasons.
- Soil inversion consistently out-yielded the control, deep ripping and soil mixing treatments. Soil inversion increased grain yield by >0.6 t/ha (>17%) over the control at Yerecoin and Darkan in 2019 and 2020.
- The addition of lime did not influence yield or soil biological activity in the two seasons after mechanical soil amelioration treatments were applied.
Aims
- Compare prevalence and distribution of soil biological communities, soilborne pathogen and nematode pests in the two seasons following three mechanical amelioration treatments to a non-ameliorated control.
- Determine if the addition of lime after mechanical amelioration influences the distribution and levels of soilborne pathogen inoculum and nematode pest levels.
Introduction
Growers in Western Australia have widely adopted mechanical soil amelioration and liming to manage subsoil constraints like acidity, compaction, water repellence and herbicide-resistant weeds. Common mechanical soil amelioration techniques include soil mixing, (e.g. ripping and spading), soil inversion (e.g. mouldboarding or one-way plough) and deep ripping, all of which lead to various degrees of soil mixing, creating a changed soil profile. These actions also mix and redistribute the living components of soil but little is known about the changes in diversity, distribution and long-term survival of the soil's biology including soilborne pathogens, nematodes and weed seeds that occur in response to deep soil amelioration. Liming is known to increase take-all disease because the pathogen prefers less acidic soils but liming effects on other common pathogens and nematode pests in WA requires further investigation.
This investigation assessed changes and potential interactions in soil biology, chemistry, and the soil profile's physical properties for two growing seasons after soil amelioration, a time when the system may be in flux.
Method
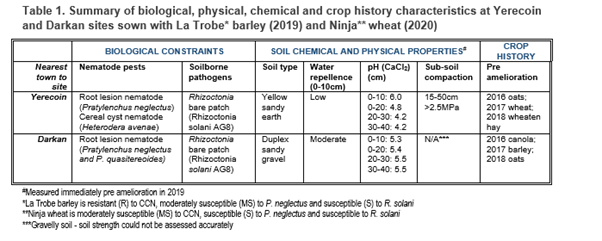
The trial was conducted at Yerecoin and Darkan, WA, in paddocks significantly impacted by soilborne disease and nematode pests and physical and chemical constraints for which mechanical amelioration is commonly undertaken (Table 1).
Deep soil amelioration
Three mechanical amelioration treatments suited to soil types at Yerecoin and Darkan were applied immediately before the 2019 cropping season. Mechanical amelioration treatments were; soil mixing (Imants 4m wide rotary spader at Yerecoin; ripping and ploughing at Darkan), soil inversion (3-furrow Kverneland mouldboard plough) and deep ripping (2m-wide Agroplow deep ripper capable of working to a depth of 45cm). All tillage implements worked to their maximum operating depth. A split-plot design was applied with post amelioration lime (+/- 2t/ha). All treatments, including a non-ameliorated control, were replicated six times, to accommodate the naturally patchy distribution of soilborne nematode and disease constraints.
Assessment of soil biological structure, nematode pests and soilborne pathogens
At the beginning of 2019 and at the end of each cropping season, we sampled soils at depths of 0-10, 10-20, 20-30 and 30-40cm and the DNA levels of the major nematode pests and soilborne pathogen present at each site (Table 1) quantified using qPCR, South Australian Research and Development Institute (SARDI). Due to COVID-19 restrictions, we only sampled the 0-10cm soil layer at the start of the 2020 cropping season. In most cases the data were square-root transformed before analyses for more constant variance as required by ANOVA (Genstat 20th Edition). The treatment structure was a factorial of amelioration (4 levels) by lime (2 levels) while the blocking structure (Rep/Amelioration) reflected the split-plot design.
At the Yerecoin site, the community structure of free-living soil nematodes was assessed to depth by qPCR post-sowing and post-harvest in 2019. This new DNA test to monitor soil biological health was applied to the same samples collected at depths outlined above post sowing and post-harvest 2019. Following the principles of nematode community structure analysis (Bongers et al 1998, Ferris et al 2001) this test measures the abundance and diversity of different nematode feeding groups as indicators of the structure and function of the soil's biological structure at the time of sampling. Multivariate methods were used for data analysis. Bray-Curtis similarity matrix was constructed to visualise the relationships between treatment soil samples and similarity percentages routine (SIMPER) was implemented to calculate similarities within and between mechanical amelioration treatments.
Results
Deep soil amelioration and crop performance
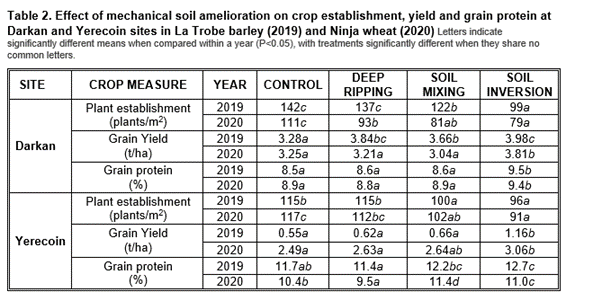
Soil amelioration affected crop establishment with soil mixing and soil inversion resulting in fewer crop plants per m2 in both years than the control for both sites (Table 2). Conversely, barley (2019) and wheat (2020) grain yield (t/ha) and protein (%) were increased after soil inversion in both years at both sites (Table 2). Soil inversion also significantly reduced weeds at both sites, indicating that this treatment buried weed seed at a depth sufficient to prevent emergence (data not shown). Deep ripping stimulated weed emergence. Therefore, soil inversion was the most effective amelioration treatment to improve crop performance at both Yerecoin and Darkan sites in the two 'transition' seasons directly after amelioration even though the sites have different soil types, constraints and environmental conditions (Tables 1 & 2). Lime did not significantly affect crop establishment or yield (data not shown).
Effect of mechanical amelioration on nematode pests and soilborne pathogen
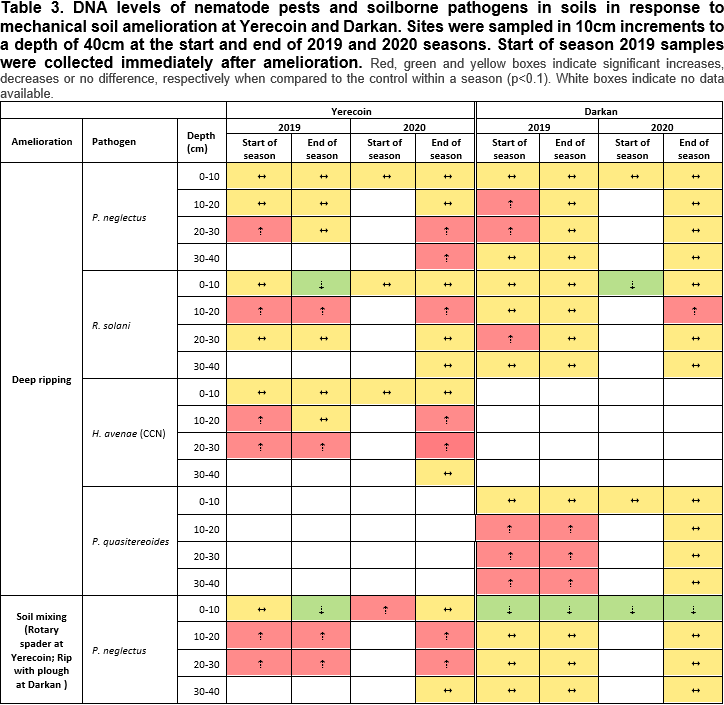
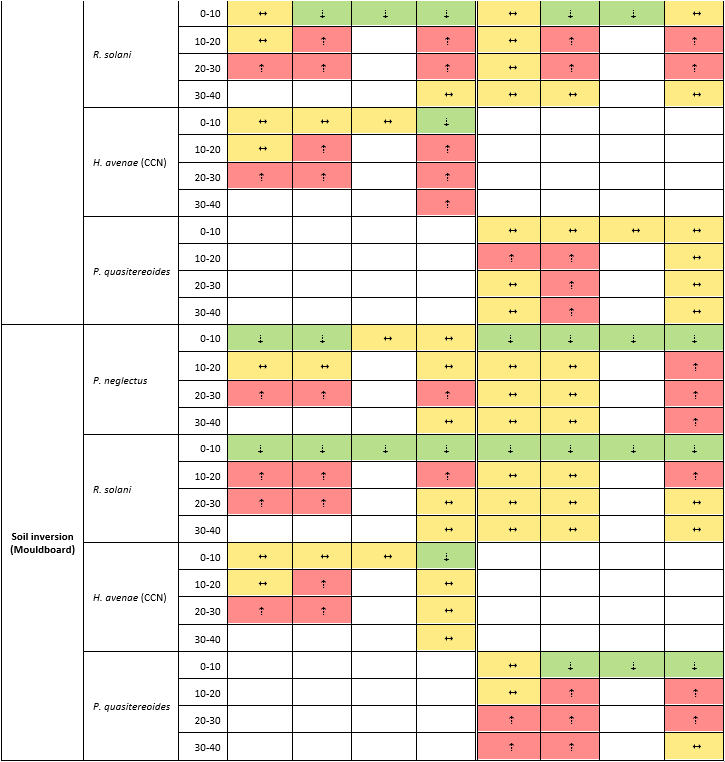
Soil amelioration generally reduced R. solani inoculum and nematode pests in the topsoil (0-10cm) but increased them at 10-40cm depth in both years and sites (Table 3). The redistribution of R. solani and nematode pests differed by the type of amelioration, with the magnitude increasing with tillage intensity in the order: deep ripping < soil mixing < soil inversion (Table 3).
Soil inversion
Soil inversion implements, such as mouldboard ploughs, bury the topsoil and bring up subsoil to the surface, with limited soil mixing occurring. Consequently, soil inversion buried the R. solani and nematode laden topsoil and brought up pathogen/pest-free subsoil to the surface at both sites (Table 3). The new topsoil remained significantly lower in R. solani than the control over the two years at the Darkan and Yerecoin sites (Table 3). Root lesion nematode (RLN) levels in the topsoil were lower at the end of 2019 season compared to non-ameliorated plots; P. neglectus at Yerecoin, P. quasitereoides plus P. neglectus at Darkan (Table 3). After two years, soil inversion continued to impact RLNs population and distribution in the profile in the Darkan duplex gravel soil with less RLN in the topsoil and the RLN surviving at depth. Impacts of soil inversion on CCN were less clear-cut. Populations in the topsoil were unaffected in the first season but lower at the end of the second.
Soil mixing
Rotary spading mixes soil within the implement's working depth, although the different layers may, to some extent, remain segregated throughout the profile. The resulting heterogeneity can be observed as patches of high organic matter content in the topsoil in a pattern that corresponds to each 'spade' action Congruent with the expected soil movement, soil mixing (rotary spading) decreased R. solani inoculum levels in the topsoil while increasing them at depth. Nematode pests were impacted similarly by soil mixing at Yerecoin but the effects were less apparent at Darkan. Soil mixing reduced P. neglectus in the top 0-10 cm at both sites by the end of 2019 season but not P. quasitereoides at Darkan (Table 3). Similar to observations with soil inversion, CCN populations in the topsoil were unaffected in the first season but lower at the end of the second.
Deep ripping
Deep ripping mechanically breaks up compacted soil layers with minimal soil mixing or soil translocation. However, some topsoil can fall into the slot behind the ripping tine and get incorporated at depth. Deep ripping redistributed both R. solani and nematode pests in the soil profile the least (Table 3), due to limited some movement occasioned by the technique. Deep ripping still increased R. solani and nematode pest levels at 10-40cm depth. Impacts on the topsoil populations of R. solani and nematode pests were minimal and transient. For example, the effects of deep ripping on R. solani levels were no longer evident two years after amelioration (Table 3). However, inoculum introduced at depth sometimes persisted over the two years.
Effect of mechanical amelioration on soil biological structure in the 1st season post amelioration
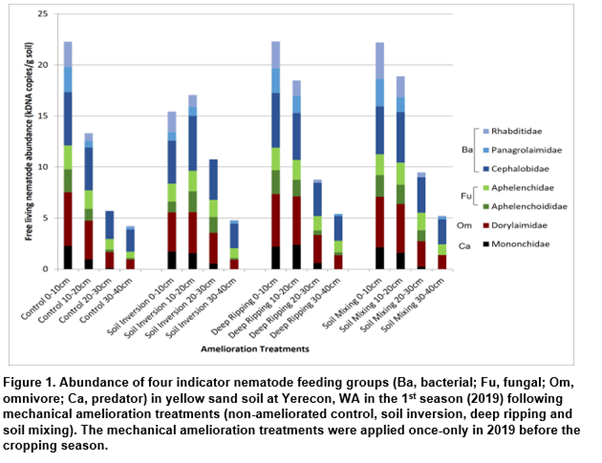
Mechanical soil amelioration had a significant impact on the presence and distribution of nematode pests and soilborne pathogens in the sandy yellow soil at the Yerecoin site. Assessment of the nematode community indicates biological activity was reduced in the topsoil (0-10cm) by soil inversion treatments and was stimulated at greater depths by all amelioration treatments, particularly between 10-30cm (Figure 1). By completion of the 1st season, amelioration had decreased pathogen levels in the topsoil and increased levels deeper in the profile. The disease implications of the changed biological communities in soil profile needs further investigation.
Conclusion
The degree of change in biological communities, including nematode pests and the soilborne pathogen that causes Rhizoctonia bare patch varied with the type of mechanical amelioration treatment. The magnitude of impacts increased with tillage intensity in the order: deep ripping < soil mixing < soil inversion. The soil movement created by inversion and mixing treatments successfully reduced R. solani and nematode pests in topsoil and increased them deeper in the profile. Deep ripping, which has much less impact on the soil profile, did not create enough soil movement to significantly impact topsoil populations.
The longevity of changes in R. solani and nematode pest levels over two seasons was dependent on site. In these trials, the nematodes moved back into the topsoil in the sandy soils at Yerecoin more rapidly than in the gravelly Darkan soils, indicating that renovation may have a longer impact on RLN in gravelly soils than in sandy soils.
R. solani, CCN and both species of RLN nematode pests investigated survived and persisted at depth. Previous studies have shown that Pratylenchus thornei,a species of root lesion nematodes can thrive in soils to a depth of 0.6m (Whish et al 2017) but this is the first record of the three pathogens investigated here persisting and multiplying at depth in WA soils. It is also the first time that our investigation has included assessment of the impact of mechanical renovation on the soil biological community. Continued research is required to improve our understanding of the mechanisms at play.
References
Bongers T, Ferris H (1999) Nematode community structure as a bioindicator in environmental monitoring. Trends in Ecology & Evolution 14 (6):224-228. doi:https://doi.org/10.1016/S0169-5347(98)01583-3
Ferris H, Bongers T, de Goede RGM (2001) A framework for soil food web diagnostics: extension of the nematode faunal analysis concept. Applied Soil Ecology 18 (1):13-29. doi:https://doi.org/10.1016/S0929-1393(01)00152-4
Whish, J. P. M., Thompson, J. P., Clewett, T. G., Wood, J., & Rostad, H. E. (2017). Predicting the slow decline of root lesion nematodes (Pratylenchus thornei) during host-free fallows to improve farm management decisions. European Journal of Agronomy, 91, 44-53. doi:https://doi.org/10.1016/j.eja.2017.09.012
Acknowledgments
Funding for this work was provided by DPIRD, GRDC and DPIRD Science partnerships. We would like to thank Russell Prowse and Todd Duggan for their patience, expert advice and allowing us to put trials on their properties. We would also like to thank the DPIRD RSUs for managing our trials: Wongan Hills; Shari Dougall and Bruce Thorpe, and Katanning RSU; Russell Quartermaine and Daniel Cox. Catherine Borger and Sultan Mia, thanks for continued expertise and collaboaration at these trial sites. Lastly, thanks to our team of casuals who provided technical support; particularly Cameron Lewis and Jono Swift.
Contact details
Sarah Collins
Department of Primary Industries and Regional Development, Western Australia
3 Baron-hay Court, Kensington. 6551.
Phone: 08 9368 3612
Mobile: 0404488113
Email: sarah.collins@dpird.wa.gov.au