Movement, breeding, baiting and biocontrol of Mediterranean snails
Movement, breeding, baiting and biocontrol of Mediterranean snails
Author: Kym Perry, Helen Brodie, Greg Baker (SARDI Entomology unit), Michael Nash (former SARDI member), Svetlana Micic (DPIRD, WA) and Kate Muirhead (SARDI Entomology unit) | Date: 09 Feb 2021
Take home messages
- Extensive datasets highlight that baiting programs should be focused during March to June.
- Snails move in response to increases in relative humidity at ground level from late summer through autumn, providing early baiting opportunities.
- Rule-of-thumb guidelines for the movement of vineyard, Italian and small pointed snails were generated from analysis of time lapse video data.
- An introduced parasitoid fly, Sarcophaga villeneuveana, parasitises up to 48% of conical snails in local areas of South Australia near favourable species mixes of native vegetation.
Introduction
This paper reports selected findings from GRDC research projects focused on improving molluscicidal control (DAS00160) and biocontrol (UOA1903-014BLX (9177340), CSE00061-PYC106)) of Mediterranean pest snails. Molluscicidal baiting is an important component of integrated snail control but provides variable levels of control despite high cost (Baker et al. 2017). An introduced parasitoid fly, Sarcophaga villeneuveana, attacks two conical snail species, Cochlicella acuta and C. barbara, with limited impact to date. Developing improved management tactics for snails remains a priority to improve growers’ profitability and reduce market access risks caused by snail contamination of the grain harvest.
The GRDC project “Biology and management of snails and slugs in grain crops” (GRDC project: DAS00160, 2017–2020), led by SARDI in collaboration with DPIRD, generated new biological knowledge of pest snails and slugs, specifically their movement behaviour and reproductive activity, to assist growers to optimise the timing of baiting programs. Efficient baiting must target adult snails before most reproduction occurs. Effective baiting to ensure snails encounter pellets requires snail movement, which must be predicted before application. This project investigated the environmental triggers for mollusc movement to provide better predictive capacity. This paper presents the results for snails.
The GRDC project, “Snail biocontrol revisited – Phase II” (GRDC project: CSE00061-PYC106; 2019 – present), led by CSIRO in collaboration with SARDI, is investigating whether strains of the parasitoid fly, S. villeneuveana, sourced from Mediterranean regions more closely aligned with the geographic origins of the Australian C. acuta, can improve biocontrol of this species. Project results are presented elsewhere. New data generated by SARDI describing existing levels of biocontrol of C. acuta by S. villeneuveana in South Australia (SA) (SARDI-GRDC project: UOA1903-014BLX (9177340)) are presented here.
This paper summarises selected findings with relevance for management. Comprehensive datasets and analyses are presented elsewhere and in project final reports (Perry et al. 2020a, Perry et al. 2020b, Caron and Yonow 2020).
Snail breeding seasons
The reproductive cycles of three snail species were studied at four SA and four Western Australia (WA) locations between 2017 and 2020 for periods from 2–4.5 years. Target species were the vineyard snail (C. virgata) at three SA sites and one WA site, the white Italian snail (T. pisana) at one SA site, and the small-pointed snail (C. barbara)at three WA sites (Table 1). Nine-month datasets were collected for C. virgata and C. acuta at three additional SA sites (for brevity, not presented). Samples of about 50 adult-sized snails were collected approximately monthly, then measurements of shell height and albumen gland length (after dissection) were recorded for each individual snail, yielding observations for 12,914 snails. Snails in a reproductive state have swollen albumen glands.
The three snail species, C. virgata, T. pisana and C. barbara, demonstrated strongly seasonal reproductive cycles with breeding seasons extending from autumn to spring (Table 1). On average, the main breeding seasons were March to late September for C. virgata, late February to late July for T. pisana, and March to October, sometimes extending into late November, for C. barbara in WA (Table 1). Limited data at three SA sites (four to eight months between July 2019 and March 2020) for the conical snail, C. acuta, suggested most breeding commenced sometime after March in 2020.
For each snail species, the timing of reproductive activity varied between seasons and/or locations, reflecting that species’ activity depends, to some extent, on local environmental conditions. However, relationships between the reproductive activity and prior rainfall or other measured climate and microclimate variables (such as soil water content, soil surface wetness, and relative humidity and temperature at different heights above ground level) were not always clear, suggesting that reproductive cycles have an underlying seasonal basis. No evidence was found of significant breeding activity from late spring to summer for any snail species during this study, even when substantial movement occurred following spring or summer rainfall.
Table 1. Breeding seasons by species.
Species | Study location | Study years | Breeding season average | Breeding season range | |
|---|---|---|---|---|---|
Vineyard snail, Cernuella virgata | SA | Palmer | 2015 – 2018 | Mar to Sep | Feb/Mar to Jul/Oct |
SA | Manoora | 2015, 2017, 2018 | Mar to Oct | Mar/Apr to Oct/Nov | |
SA | Urania | 2018 – 2020 | Apr to Sep | Mar/May to Aug/Oct | |
WA | Gairdner | 2017, 2018 | Mar to Oct | Feb/Mar to Oct/Nov | |
4 sites | 12 years | Mar to Sep | |||
White Italian snail, Theba pisana | SA | Warooka | 2015 – 2018 | Feb to Jul | Jan/Feb to Jul/Aug |
1 site | 4 years | late Feb – late Jul | |||
Small-pointed snail, Cochlicella barbara | WA | Esperance Marshall | 2018 | Jan to Sep | |
WA | Esperance Perks | 2017, 2018 | Mar to Sep | Feb/Apr to Sep/– | |
WA | Woogenellup | 2017, 2018 | Mar to Nov | Mar/Apr to Nov/– | |
3 sites | 5 years | Mar to Oct | |||
Snail movement and microclimate
Movement behaviour of snails was studied at ten locations in SA and WA (seven sites in Table 1 with exception of Manoora, plus three other SA sites) between 2015 and 2020 for periods from nine months to 4.5 years. Time lapse video footage was collected continuously at 1-minute intervals and microclimate variables (e.g., soil water content at 10 cm depth, soil surface wetness, ground level relative humidity and temperature, and others) were logged at 30-minute intervals. Video footage was analysed using computer vision techniques developed by collaborators at University of South Australia, yielding 103,228,235 observations of individual movement distance per frame. Manual ground-truthing estimated that autodetection accuracy was approximately 85% for the round snail species but less than 40% for the small-pointed snails due to greater detection challenges. Movement data were statistically analysed to determine microclimate conditions that best explained low or high snail movement at different times of the year.
In general, snails became increasingly responsive (moved) to increases in ground level relative humidity from late summer through autumn. Other microclimate variables and interactions between variables were associated with high/low movement, however these relationships were less clear (Perry et al. 2020b). For simplicity, rule-of-thumb guidelines for snail movement with respect to relative humidity were generated from the data (Table 2). These guidelines are simply a set of hypotheses generated from the available data and should be tested and refined over time under field conditions. There is greater confidence in the information for the round snails, C. virgata and T. pisana, than for the small-pointed snails, based on higher detection accuracy.
Table 2. Rule-of-thumb levels of relative humidity at ground level associated with the highest observed movement.
Species | Feb | Mar | Apr | May | Autumn |
|---|---|---|---|---|---|
Vineyard snail | > 95 % | > 90 % | > 80–85 % | > 85–95 % | |
Italian snail | > 90 % | > 90 % | > 85–90 % | > 88 % | |
Small-pointed snail | > 95 % | > 95 % | > 95 % | > 95 % | |
Implications for bait timing
All datasets together highlighted that baiting programs targeting C. virgata, T. pisana, and C. barbara should be concentrated during the autumn and early winter period, from approximately March to June, prior to most reproduction, to maximise cost-efficiency. There are several reasons for this recommended timing:
- Snails showed higher susceptibility to bait toxins during this period than during non-reproductive periods (Brodie et al. 2020, Perry et al. 2020b, and presentation slides).
- Snails feed voraciously on baits immediately after exiting summer aestivation.
- Most offspring are produced during the early phase of the breeding season; Targeting adult snails before most eggs are laid minimises offspring production.
- Baiting prior to crop sowing minimises soil surface obstacles and alternative food sources (e.g., crop seedlings), thereby increasing the chance of bait encounter.
It is recommended that growers commence monitoring for baiting opportunities from late summer, approximately February onwards, as snails move opportunistically in response to increased moisture or relative humidity at this time. Baiting from January or earlier is likely to be less efficient because:
- Snails may be less susceptible to bait toxins during this period compared with their reproductive periods.
- Exposure of bait pellets to high temperatures (>35 oC) can cause loss of active ingredient (Baker et al. 2017).
- Baiting too early increases the chance of killing some snails that would otherwise die naturally from heat/dry stress (e.g., Perry et al. 2020a), thereby wasting bait.
It is suggested that baiting programs should generally cease by mid-winter or earlier as later applications are less efficient. Instead, baits should be used earlier in the season or in the following season during the optimal windows.
Time lapse video showed that initial increases in movement during late summer through autumn occurred mostly overnight (not shown). To detect this movement and confirm whether snails are feeding, growers can deploy small areas of bait in infested areas prior to widespread application.
Biocontrol of conical snails
The fly, Sarcophaga villeneuveana, is a specialist parasitoid of the conical snail, C. acuta and small-pointed snail, C. barbara. Strains of S. villeneuveana were sourced from the Montpellier region, of France, and introduced into SA by SARDI and CSIRO between 2001–2004 for biocontrol of C. acuta (Leyson et al. 2003). The fly successfully established on southern Yorke Peninsula but exhibited limited spread and impact, with pre-2018 levels of C. acuta parasitism estimated at less than 2% (SARDI unpublished). A current GRDC project (CSE00061-PYC106, 2019–present), conducted by CSIRO and SARDI, has focused on enhancing biocontrol success by introducing S. villeneuveana sourced from areas of Spain and Morocco, better matching the geographic origins of Australian C. acuta (Jourdan et al. 2019). In 2020, Moroccan fly strains were imported by CSIRO and reared in quarantine facilities at SARDI for evaluation of host specificity prior to seeking approval for a rear-release program.
To enable assessments of the impact of future fly releases, SARDI generated baseline data on the current level of conical snail parasitism by S. villeneuveana (project: UOA1903-014BLX (9177340)). In January and April of 2019 and 2020, C. acuta and C. barbara were collected from 19 sites on Yorke Peninsula and from four different microhabitats:
- Ground-level, in quadrats;
- elevated (e.g., on plants, stubble and fence posts);
- at the base of tussocks, plants and grasses; and
- under refuges (e.g., logs and rocks).
Snails were returned to the laboratory, reared and examined for parasitism.
From 85,673 C. acuta and 2,412 C. barbara of suitable size (> 5mm) assessed for parasitism, S. villeneuveana was detected in snails from 13/19 sites (Figure 1). At sites where S. villeneuveana was detected, overall parasitism was 2.8% for C. acuta and 3.4% for C. barbara. Mean parasitism rates were significantly higher for C. acuta snails on elevated substrates (10.8%) than at the base of plants (4.1%), at ground level (4.4%) or under refuges (1.7%) (Figure 2). At individual sites and sampling dates, parasitism ranged from 0–48% for C. acuta and 0–27% for C. barbara. Higher parasitism levels were observed at sites adjacent to native vegetation flowering during periods of fly activity (spring/summer), suggesting vegetation provides food and/or shelter resources.
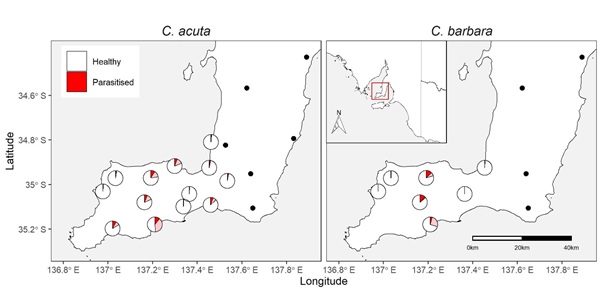
Figure 1. Parasitism levels of conical C. acuta and C. barbara by the parasitoid fly, S. villeneuveana. Pies show the proportion mean overall parasitism (dark shading) or maximum parasitism observed on a single sampling date (light shading) at sites where S. villeneuveana was present, while black dots indicate absence of S. villeneuveana at a sampled site.
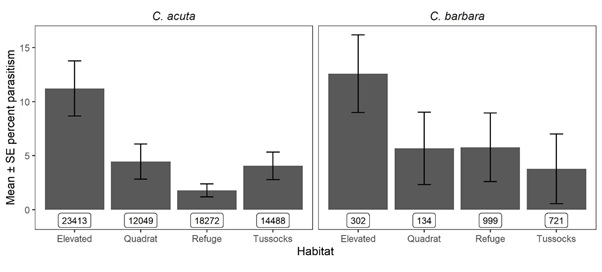
Figure 2: Parasitism of conical snails by S. villeneuveana in four microhabitats in 2019 and 2020. Sample sizes per category are shown in boxes.
Conclusions
Findings from DAS00160 generated a sound evidence base underpinning best practice snail management and provided growers with new information to refine their baiting strategies. Additionally, novel infrastructure (methods, analyses) for mollusc movement studies were also developed for future use. Further development is required to improve computer vision detection accuracy for conical snail species, and to generate deeper understanding of their movement and management. It was discovered that the introduced parasitoid fly, S. villeneuveana, performs well in the Yorke Peninsula climate in local areas with suitable habitat. Furthermore, S. villeneuveana attacks C. barbara at similar rates to C. acuta and is therefore suitable for release in other regions (e.g., Western Australia) for biocontrol of either species.
Acknowledgements
This research was made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC. The authors thank them for their continued support. Ivan Lee and colleagues (University of South Australia) developed and implemented the computer vision analysis of movement data. Statistical analysis was performed by SAGI South (University of Adelaide, Biometry Hub).
References
Baker GJ, Brodie H, Nash MA, Cunningham N, Perry KD (2017). Improved management of snails and slugs. Final report for GRDC (DAS00134). South Australian Research and Development Institute.
Brodie H, Baker GJ, Muirhead K, Perry KD (2020). “Snails: learnings from recent studies”. Proceedings paper, GRDC Grains Research Updates, Adelaide 11–12 February 2020.
Caron V and Yonow T (2020). Snail biological control revisited – Phase 2. Progress report for GRDC (CSE00061-PYC106). CSIRO.
Jourdan M, Thomann T, Kriticos DJ, Bon MC, Sheppard A, & Baker GH (2019). Sourcing effective biological control agents of conical snails, Cochlicella acuta, in Europe and north Africa for release in southern Australia. Biological Control, 134, 1-14.
Leyson M, Hopkins DC, Charwat S, Baker GJ (2003). Release and establishment in South Australia of Sarcophaga penicillata (Diptera: Sarcophagidae), a biological control agent for Cochlicella acuta (Mollusca: Hygromiidae). In: Dussart, G.B.J. (Ed.) Slugs and Snails: Agricultural, Veterinary & Environmental Perspectives. 295–300. Proceedings 80th BCPC Symposium, British Crop Protection Council, Thornton Heath, U.K.
Perry KD, Brodie H, Muirhead K (2020a). New methods for snail control. Final report for GRDC and SARDI (UOA1903-014BLX (9177340)). South Australian Research and Development Institute; University of Adelaide.
Perry KD, Brodie H, Fechner N, Baker GJ, Nash MA, Micic S, Muirhead K (2020b). Biology and management of snails and slugs in grains crops. Final report for GRDC (DAS00160). South Australian Research and Development Institute.
Contact details
Dr Kym Perry
SARDI, Waite Campus, Urrbrae SA 5064
088429 0738 | 0421 788 357
kym.perry@sa.gov.au
GRDC Project Code: DAS00160, UOA1903-014BLX (9177340), CSE00061-PYC106,
